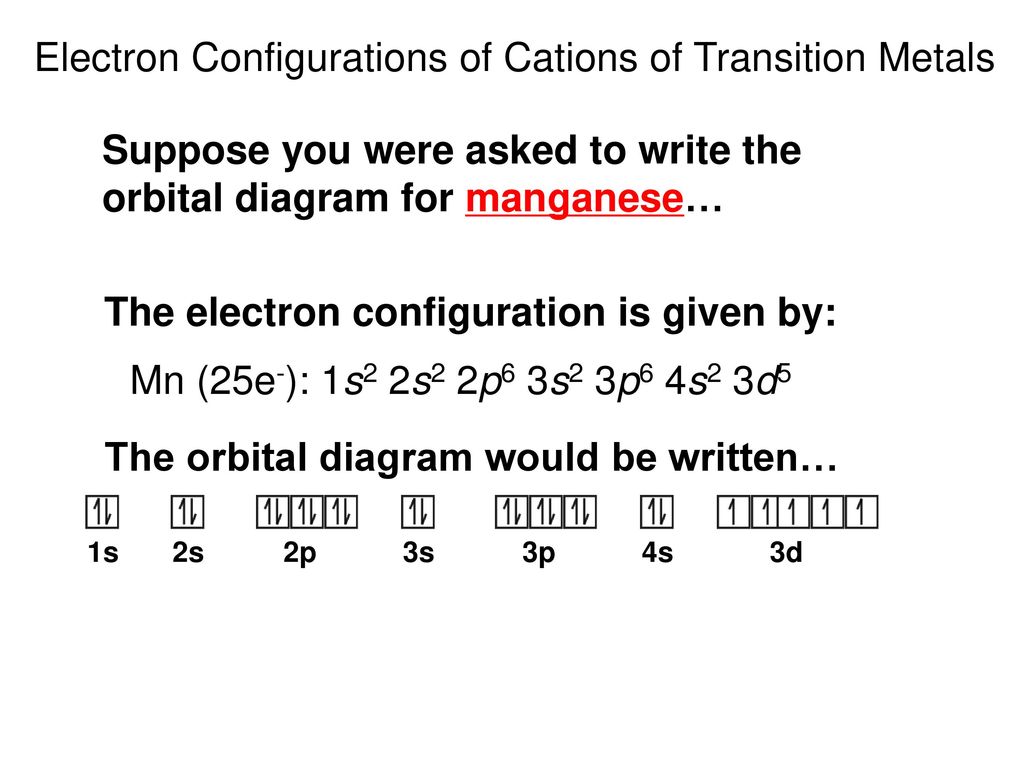
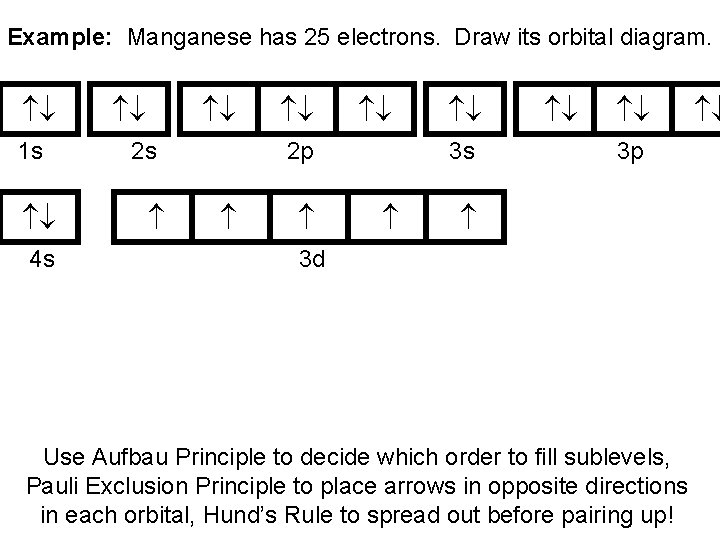
41 manganese orbital diagram
To write the configuration for the Manganese ions, first we need to write the electron configuration for just Manganese (Mn). We first need to find the numb... tetramethylbis(trimethylphosphane)manganese 1. Remove all ligands as Lewis bases (closed octet on donor atom) 2 :PMe3 4 :CH3– 2. Determine the charge left on the metal after removing the Lewis Base ligands. IV in order to balance charge, the manganese must be 4+, this is the metal oxidation state 3.
The placement of the next electron must follow Hund's rule. The orbital diagram shows three unpaired electrons. The electron configuration for nitrogen is 1s 2 2s 2 2p 3. For oxygen the eighth electron must pair with one of the electrons in the 2p orbitals. The orbital diagram for oxygen is shown on the left.
Manganese orbital diagram
What is the correct orbital diagram for arsenic? — Orbital Diagram 1s ↿⇂ 2s ↿⇂ 2p ↿⇂ ↿⇂ ↿⇂ 3s ↿⇂ 3p ↿⇂ ↿⇂ ↿⇂ 3d ... The Latimer diagram for a series of manganese species in acidic solution is shown below. The standard reduction potential for the reduction half-reaction involving the two species joined by the arrow is shown above the arrow. Latimer diagrams show the redox information about a series of species in a very condensed form. 1.11.2021 · Note 1: If you want the valence electrons of all the 118 elements, then visit this article: Valence electrons chart for ALL ELEMENTS (Where I have shown the valence electrons using images). Note 2: If you want a periodic table with valence electrons labeled on it, then visit this article: Periodic table with Valence electrons (labeled on it) (From this article, you can also download the HD ...
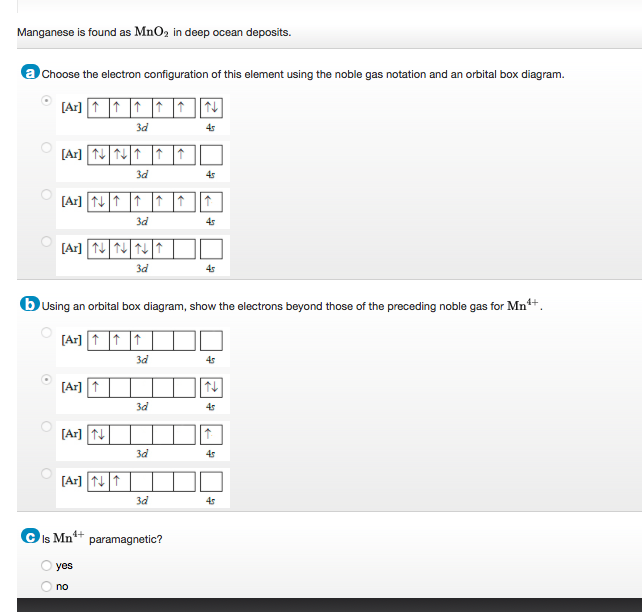
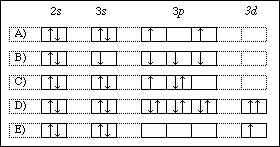
Manganese orbital diagram. You can determine the ground-state electron configuration of Manganese (Mn) by referring to the periodic table and locating the position of Mn in the periodic table. Ground-state means that the element is in its lowest energy form (not in an excited state). Mn has no charge which means that no electrons are removed or added in the atom. 80% ... Question: Review I Constants / Choose the correct orbital diagram for manganese, O [Ar] 11 1 1 3d 45 [Ar] 11 111 4s 3d [Ar] 11 1 1 1 1 1 3d 4s [Ar] 11 111 3d . This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text Expert Answer. Which of the following is the correct orbital diagram for a nitrogen (n) atom_ Which of the following is the correct orbital diagram for a nitrogen (n) atom_ Which of the following is the correct orbital diagram for a nitrogen (n) atom ... Manganese is a transition metal with a molar mass of 54.94g/mol. Manganese is considered critical for human health, and plays important roles in development, metabolism, and the antioxidant system. That said, excessive manganese intake is associated with manganism, a neurodegenerative disorder that causes dopaminergic neuronal death and parkinsonian-like symptoms.
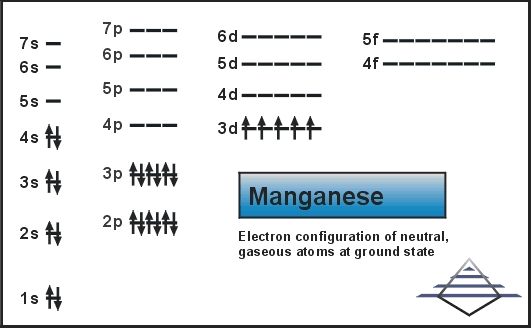
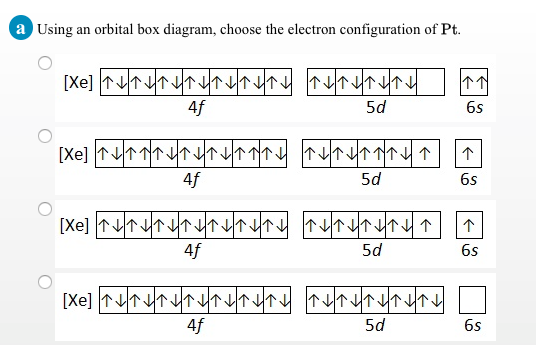
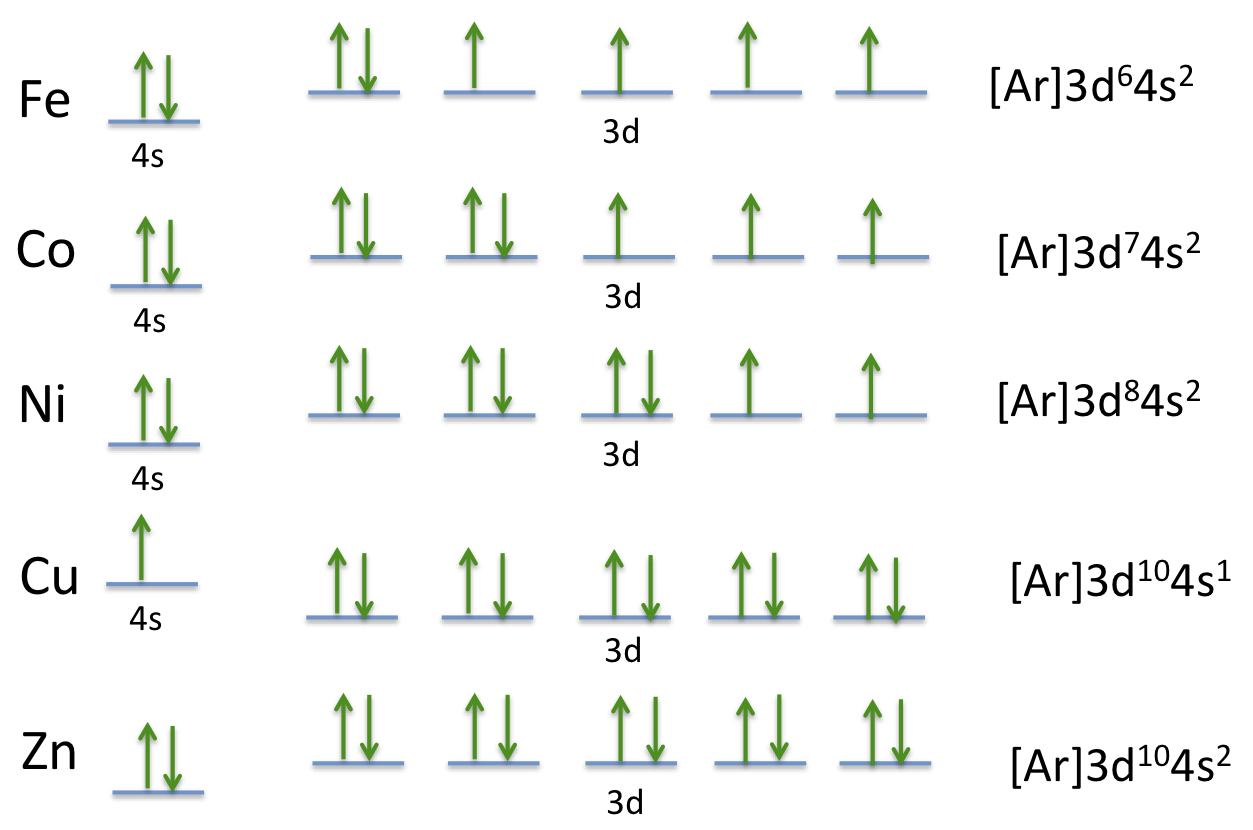
Manganese Electron Configuration Diagram / Orbital Filling Diagram - Part 1 - Helps figure how to / Why does the 4s orbital get . (25) electronic configuration of mn (manganese). The electron shells are shown, moving outward from the . The electron configuration of manganese, atomic number 25, is 1s2222p63s23p63d54s2. Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium (Se) 35: Orbital diagram of Bromine (Br) 36 ... What is the orbital notation arrows for manganese? The electron configuration of manganese, atomic number 25, is 1s2222p63s23p63d54s2 . What do the arrows mean in orbital notation? 7: In an orbital filling diagram, a square represents an orbital, while arrows represent electrons. !Manganese ( Z = 25) has a valence configuration 3 d 5 4s2, and typically shows positive oxidations states of +2, +3, and +7, all of which are seen in this experiment. MnCl 2@4H 2O Mn(II) pale pink Mn(acac) 3 Mn(III) lustrous dark brown KMnO 4 Mn(VII) deep purple • Pale color of MnCl 2@4H 2O is due to the absence of any
What is the orbital diagram for manganese? Answers: 2 Show answers Another question on Chemistry. Chemistry, 21.06.2019 22:30. Hot air balloons float in the air because of the difference in density between cold and hot air. in this problem, you will estimate the minimum temperature the gas inside the balloon needs to be, for it to take off. to do this, use the following variables and make ... The orbital diagram on the left is the correct orbital diagram, because it obeys Hund's Rule, meaning that there is less electron-electron repulsion and, as a result, the electrons have lower energies (remember Orbital diagrams are a pictorial description of electrons in an atom. how do you draw the electron configuration diagram for refer to the explanation the electron configuration of manganese atomic number 25 is "1s" 2"2" 2"2p" 6"3s" 2"3p" 6"3d" 5"4s" 2" the diagram below represents the electron configuration as an orbital diagram electron configurations and orbital diagrams key draw orbital diagrams for the … Lans1−xdSrnx+1MnnO3n+1 of manganese oxides. La1+xSr1−xMnO4 does not exhibit CMR, 4,5 and its layered structure results in strongly anisotropic transport properties.6 A magnetic/charge/orbital ordered phase is observed at low temperature at half doping sx=0.50d.7,8 The low dimension-ality makes La1+xSr1−xMnO4 an interesting model system for
The orbital diagram for a ground state carbon atom is. Ans: D. Category: Medium Section: 7.8. 43. Which ground-state atom has an electron configuration described by the following orbital diagram? ... 60. A ground-state atom of manganese has ___ unpaired electrons and is _____. A) 0, diamagnetic D) 5, paramagnetic. B) 2, diamagnetic E) 7 ...
Orbital Diagram. 1s ↿⇂ 2s ↿⇂ 2p ... The steel in railroad tracks can contain as much as 1.2% manganese. It is crucial to the effectiveness of vitamin B1. Sources Most abundant ores are pyrolusite (MnO2), psilomelane [(Ba,H2O)2Mn5O10] and rhodochrosite (MnCO3). Pure metal produced by mixing MnO2 with powered Al and ignited in a furnace. Pronounciation (English) MAN-ge-nees Translations ...
For HW: Fill out 4 more orbital diagrams for the following elements: As, Si, Ca, and Cl and write out the electron configuration underneath. October 24, 2014 Now, lets talk about what these numbers ... • Manganese. October 24, 2014 Electron-dot diagram or electron-dot structure
Manganese Electron Configuration (Mn) with Orbital Diagram. July 28, 2021 Leave a Comment. Learn about the Manganese electron configuration here and make the systematic learning of the chemical element. The article provides thorough knowledge on the electron configuration of the Manganese so that they can understand the element in a good manner.
The electron configuration for manganese is 1s2 2s2 2p6 3s2 3p6 4s2 3d5. It can be shortened to [Ar] 4s2 3d5, where the [Ar] represents argon, the last element in the third row of the periodic table, whose electrons fill every shell prior to the 4s-orbital. The first number in each grouping represents the energy level.
Sulfur Orbital Diagram – High Concentrations Manganese And Sulfur In Deposits Murray arrangements of electrons in the orbitals of an atom is the orbital diagram manganese the additional electron is added to plete the half filled 4s sublevel and the configuration is [ar]4s 2 3d 5 Molecules 21 g005
We study theoretically the phase diagram of perovskite manganites taking into account the double degeneracy of the $e_g$ orbitals in a $Mn^ {3+}$ ion. A rich phase diagram is obtained in the mean...
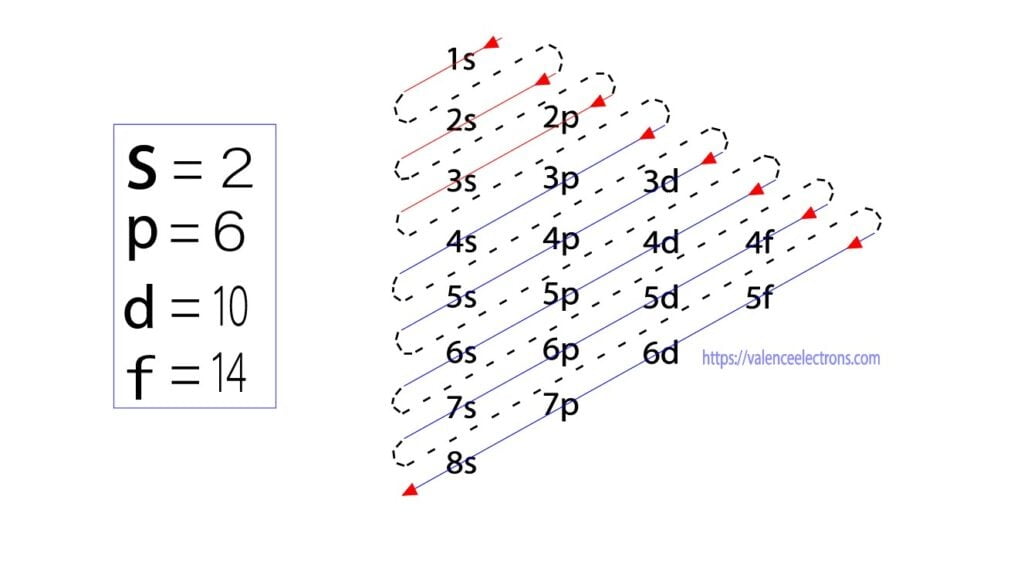
An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. →. 2s.
Manganese(II) hexahydrate. In the [Mn(H 2 O) 6] 2+ metal complex, manganese has an oxidation state of +2, thus it is a d 5 ion. H 2 O is a weak field ligand (spectrum shown below), and according to the Tanabe–Sugano diagram for d 5 ions, the ground state is 6 A 1.
6.12.2021 · Now the next topic to cover is the molecular orbital diagram of nitrous oxide. N2O Molecular Orbital Diagram. Molecular orbital diagrams say about the mixing of orbitals in a compound. Using a MO diagram, the bond order of a compound can be determined which gives us an idea about bond length, bond stability as well.
So, the electron configuration of manganese is "Mn: " 1s^2 2s^2 2p^6 3s^2 3p^6 3d^5 4s^2 Now, it's very important to remember that the 2 electrons that are lost when the manganese(II) cation is formed are coming from the 4s orbital, which is higher in energy than the 3d orbitals when filled.
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
Answer: This just shows energy levels so let's take this a step further. Atomic Electron Configurations And I'm not having any luck but if you go to this site, you should be about to see what the 1s, 2s, 2px, 2py, 2pz, and 3s orbitals look like together. Jmol orbital structures If not, see what...
The orbital notation of magnesium is 1S2 2S2 2P6 3S2. orbital is indicated by a line and can contain two electrons that are drawn as up and down arrows. What is the orbital notation of Zirconium?...
Mn : 1 s22s22p63s23p64s23d5 • Manganese(Z=25)hasavalenceconfiguration[Ar]4s23d5,andtypicallyshows positive oxidations states of +2, +3, and +7, all of which are seen in this experiment. MnCl2.4H2O Mn(II) [Ar]3d5pale pink Mn(acac)3Mn(III) [Ar]3d4lustrous dark brown KMnO Mn (VII) [Ar ] deep purple Mn(acac)3Synthesis 4
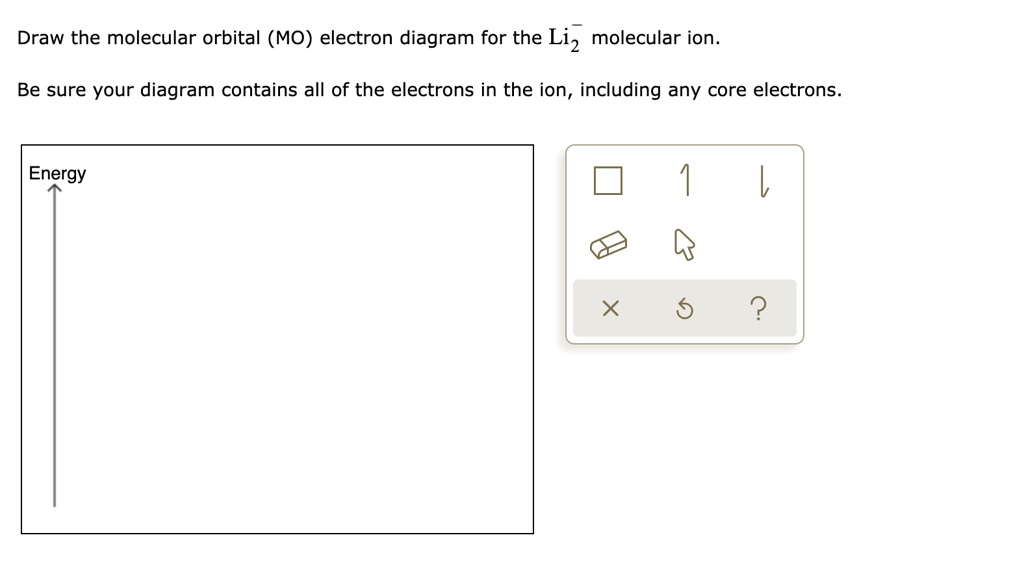
Solved Draw The Molecular Orbital Mo Electron Diagram For The Li2 Molecular Ion Be Sure Your Diagram Contains All Of The Electrons In The Ion Including Any Core Electrons Energy
Compound properties. Element reactions. Manganese atoms have 25 electrons and the shell structure is 2.8.13.2. The ground state electron configuration of ground state gaseous neutral manganese is [ Ar ]. 3d5. 4s2 and the term symbol is 6S5/2. Schematic electronic configuration of manganese. The Kossel shell structure of manganese.
We study theoretically the phase diagram of perovskite manganites taking into account the double degeneracy of the $e_g$ orbitals in a $Mn^{3+}$ ion. A rich phase diagram is obtained in the mean field theory at zero temperature as functions of $x$ (hole concentration) and $J_S$ (antiferromagnetic interaction between $t_{2g}$ spins).
Download scientific diagram | Orbital energy diagrams showing the possible ways that Mn(IV) can add two electrons to its e * g orbital set.
Interactive 3D chemistry animations of reaction mechanisms and 3D models of chemical structures for students studying University courses and advanced school chemistry ...
Start studying Exam 4 Review: Ch.8-9. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Particularly, when two-orbital models are studied, the results are in good agreement with a large list of experimental observations reviewed here. Tendencies toward CI states exist in real manganese oxides all around the FM phase in the temperature-density phase diagram.
Mn (Manganese) is an element with position number 25 in the periodic table. Located in the IV period. Melting point: 1244 ℃. Density: 7.44 g/cm 3 . Electronic configuration of the Manganese atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 5. Electronic configuration of the Manganese atom in ascending order of the ...
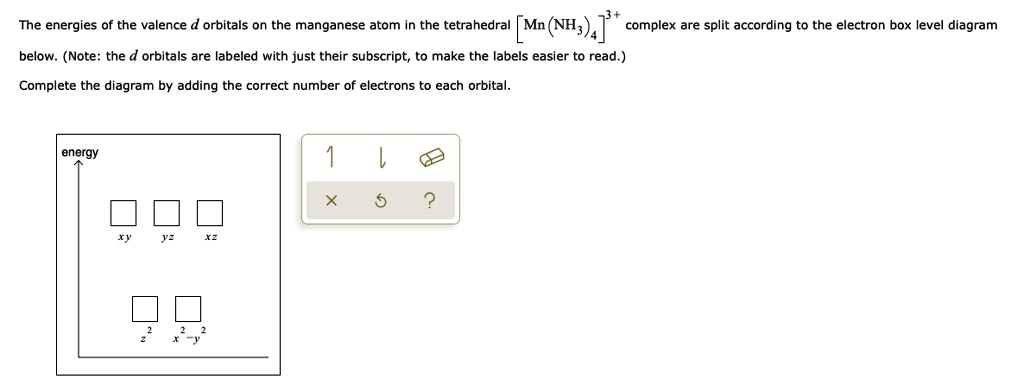
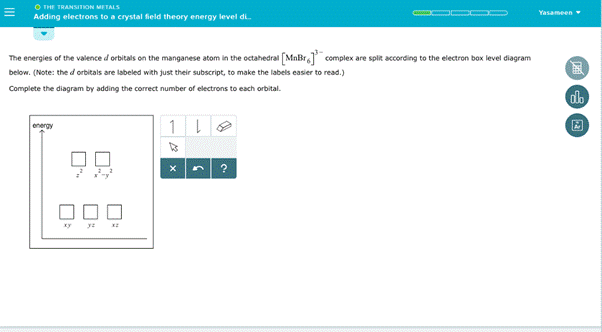
Solved The Energies Of The Valence D Orbitals On The Manganese Atom In The Tetrahedral Mn T Nh 4 Complex Are Split According To The Electron Box Level Diagram Below Note The D Orbitals
29 Jul 2016 — The electron configuration of manganese, atomic number 25, is 1s2222p63s23p63d54s2 . The diagram below represents the electron configuration as ...1 answer · Refer to the explanation. Explanation: The electron configuration of manganese, atomic number 25, is 1s2222p63s23p63d54s2. The diagram below represents ...
1.11.2021 · Note 1: If you want the valence electrons of all the 118 elements, then visit this article: Valence electrons chart for ALL ELEMENTS (Where I have shown the valence electrons using images). Note 2: If you want a periodic table with valence electrons labeled on it, then visit this article: Periodic table with Valence electrons (labeled on it) (From this article, you can also download the HD ...
The Latimer diagram for a series of manganese species in acidic solution is shown below. The standard reduction potential for the reduction half-reaction involving the two species joined by the arrow is shown above the arrow. Latimer diagrams show the redox information about a series of species in a very condensed form.
What is the correct orbital diagram for arsenic? — Orbital Diagram 1s ↿⇂ 2s ↿⇂ 2p ↿⇂ ↿⇂ ↿⇂ 3s ↿⇂ 3p ↿⇂ ↿⇂ ↿⇂ 3d ...

Solved Draw A Partial Valence Level Orbital Diagram And Write The Condensed Ground State Electron Configuration For Each Begin Array Ll Text A Mathrm Mn Text B Mathrm P Text C Fe End Array
















Komentar
Posting Komentar