38 lewis dot diagram for hcn
Can someone please help me figure out how to draw the Lewis dot diagram for a Na- anion? Thank you. Draw the Lewis dot structure of Hydrogen cyanide (HCN) molecule . Updated On: 14-12-2020. check-circle. Answer. Step by step solution by experts to help you ...2 answers · Top answer: Solution:
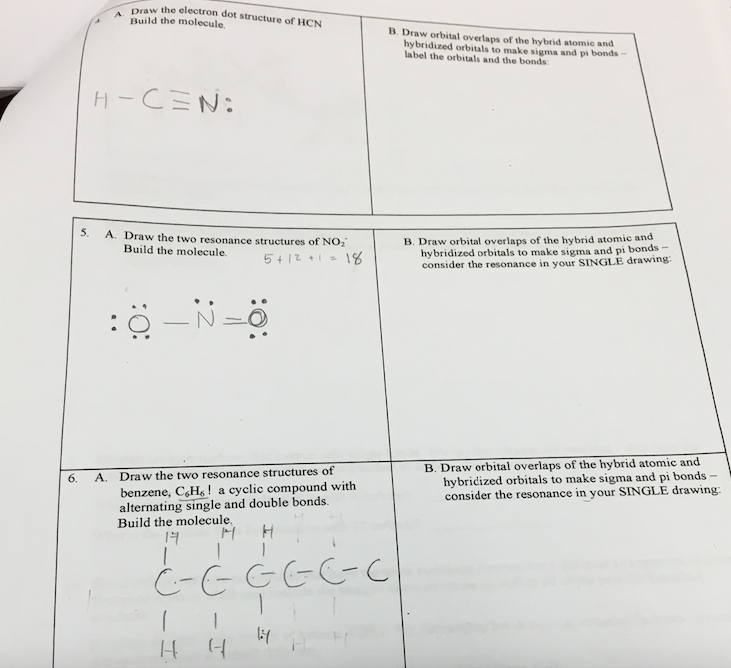
Nov 12, 2017 — Explanation: · Step 1. Draw a skeleton structure · Step 2. Count the valence electrons you can use · Step 3. Add these electrons to give every atom ...1 answer · Here's how to do it. Explanation: Step 1. Draw a skeleton structure Put the least electronegative atom C in the middle with H and Cl on either side. ...

Lewis dot diagram for hcn
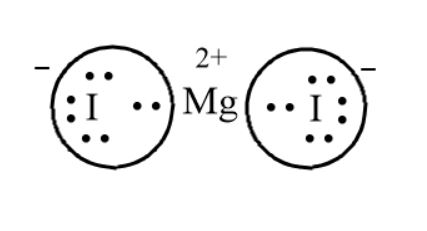
Hi, I am working on a project in which we need to draw the Lewis Dot Structure for five binary molecular compounds. Although (I think) I understand how to do these with normal elements, I am confused on how to do it with compounds. The teacher has allowed us to search these up (it is one small part of the project), but I am unable to find or understand what comes up. ​ Examples: Triphosphorus pentanitride (P3N5) Searching up brings up images that contain more than 3/5 of each el... I am having trouble with a question about a lewis dot diagram. If phosphorus and bromine atoms formed a compound, what would the lewis dot diagram and chemical formula be? Would the chemical formula be: PBr? (I couldn't find the formula anywhere on the internet to confirm if the formula is simply PBr). If it is not simply PBr, why or what makes it something else? If it is just PBr, would the lewis diagram be a single bond? \-I think I did it correctly, just wanting to make sure. Jan 13, 2020 — Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams. ... It is written in the following steps :2 answers · Top answer: Solution: It is written in the following steps : Step I. Total number f valence electrons ...
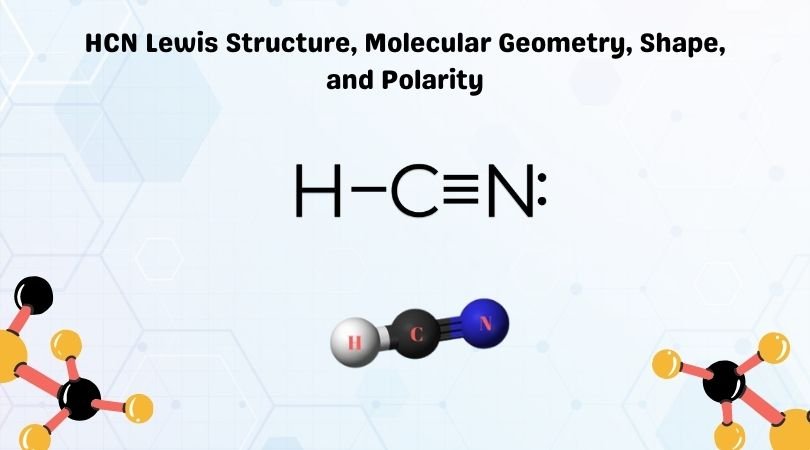
Lewis dot diagram for hcn. Recently we began a chapter in Chemistry where we had to find the Lewis Dot Structures of Molecules (I.e. using valence electrons, adding in bonds, lone pairs, etc.) and I simply cannot get it. The process and sequence to get to the finished product is confusing, which is unfamiliar to me, as I am someone who typically very easily grasps concepts. I would very much appreciate anyone who could shed some light on their ways of thinking, easier way to go about it, or other tips, as my teacher has e... If you have a link or image of one you can send to me that would be appreciated! If anyone can give me an explanation or help me understand how it will look. Once you get the total number of valence electrons, you can make a Lewis dot structure of HCN. This structure helps in understanding the ...Jul 23, 2021 · Uploaded by Geometry of MoleculesHCN valence electrons · HCN Lewis structure · HCN Molecular Geometry
Could some one please explain to me why [this](http://www.wolframalpha.com/input/?i=Thiocyanate&a=*C.Thiocyanate-_*ChemicalIntermediate-) sulfur has a negative charge? Thank You. (•‿•) I'll forever be in your debt We have a total of ten valence electrons for the HCN Lewis structure. We'll put two between atoms to form chemical bonds, so we've used four, then we'll go ...Oct 1, 2013 · Uploaded by Wayne Breslyn Shouldn't oxygen have two bonds? or does the negative outside the brackets signify that both oxygen and hydrogen gain that one electron? Am I thinking about this properly?!
lewis dot diagram for sodium hypochlorite Hello! Can someone please explain to me how to draw the Lewis diagrams for transition metals? I understand how to find the valence electrons based on the electron configuration. For elements 27+, they begin having more than 8 valence? Please help I am confused! Jan 13, 2020 — Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams. ... It is written in the following steps :2 answers · Top answer: Solution: It is written in the following steps : Step I. Total number f valence electrons ... I am having trouble with a question about a lewis dot diagram. If phosphorus and bromine atoms formed a compound, what would the lewis dot diagram and chemical formula be? Would the chemical formula be: PBr? (I couldn't find the formula anywhere on the internet to confirm if the formula is simply PBr). If it is not simply PBr, why or what makes it something else? If it is just PBr, would the lewis diagram be a single bond? \-I think I did it correctly, just wanting to make sure.
Hi, I am working on a project in which we need to draw the Lewis Dot Structure for five binary molecular compounds. Although (I think) I understand how to do these with normal elements, I am confused on how to do it with compounds. The teacher has allowed us to search these up (it is one small part of the project), but I am unable to find or understand what comes up. ​ Examples: Triphosphorus pentanitride (P3N5) Searching up brings up images that contain more than 3/5 of each el...







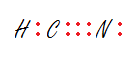





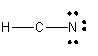



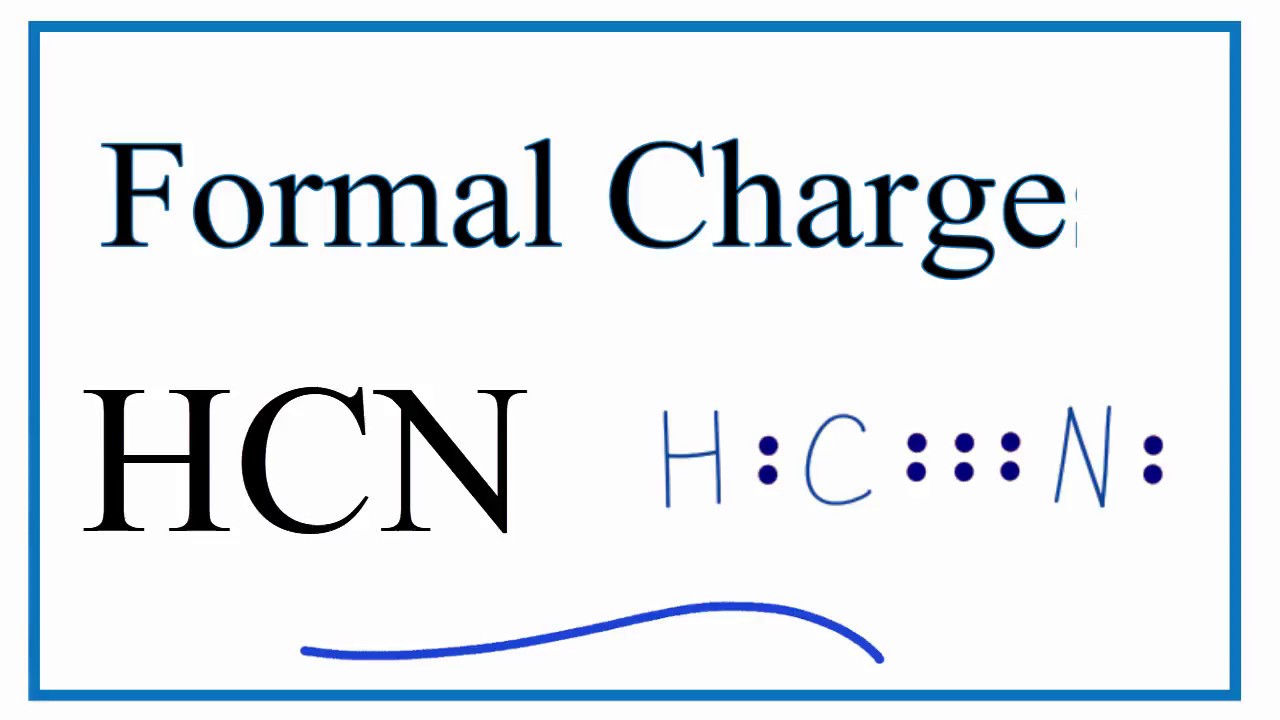





Komentar
Posting Komentar