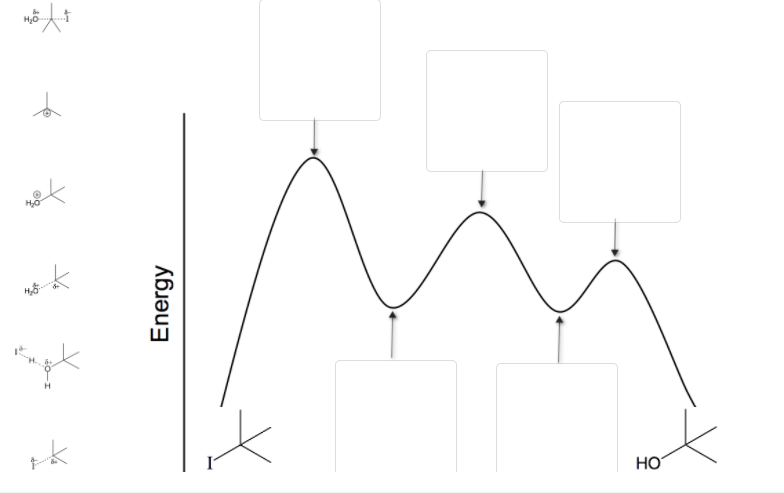
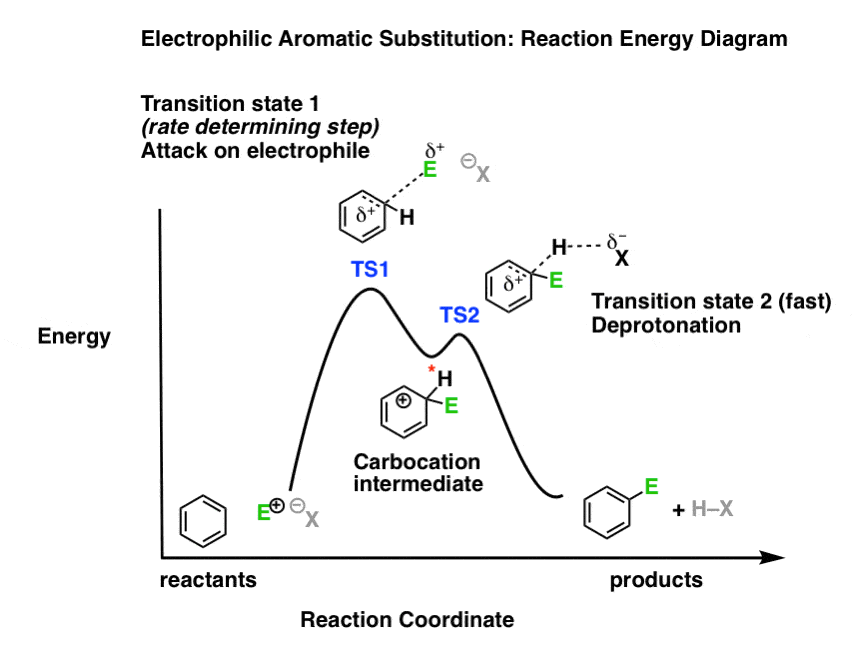
43 where are the intermediates and transition states in this diagram?
The transition state is the point of highest energy between the reactant(s) and product(s). It is not a stable molecule and can't be isolated. After the transition state, the energy of the system gradually decreases as the high energy structure relaxes and becomes product(s), B. The energy diagram for the catalysis indicates that electronic effects are responsible most of all for the relative reduction of energy of the intermediates and possibly transition states on the catalytic reaction path. The shape of the diagram qualitatively agrees with the transition-state analogue theory for the enzymatic reactions. PMID: 9007691
Question: Where are the intermediates and transition states in this diagram? Drag the appropriate labels to their respective targets. Note: not all targets will be used. ANSWER Reset Help Uncatalyzed reaction Catalyzed reaction 2 H intermediate transition state 2 H2O2 2 Br +2 H 2 H2O O2 2 H2O +O2+ 2 Br 2 H+ Reaction progress.
Where are the intermediates and transition states in this diagram?
Draw a graph showing the reaction pathway for an overall exothermic reaction with two intermediates that are produced at different rates. On your graph indicate the reactants, products, intermediates, transition states, and activation energies. Learn this topic by watching Energy Diagram Concept Videos. Transition States vs. Intermediates! A transition state is an unstable species! (it has no measurable lifetime)! Reaction Coordinate! An intermediate has a measureable lifetime! (it can be isolated in theory)! The energy of activation refers to the energy difference between the starting material and the transition state along the reaction ... May 30, 2020 — ... Reaction Energy Diagram to discuss transition states, Ea, intermediates & rate determining step; draw the transition state of a reaction.
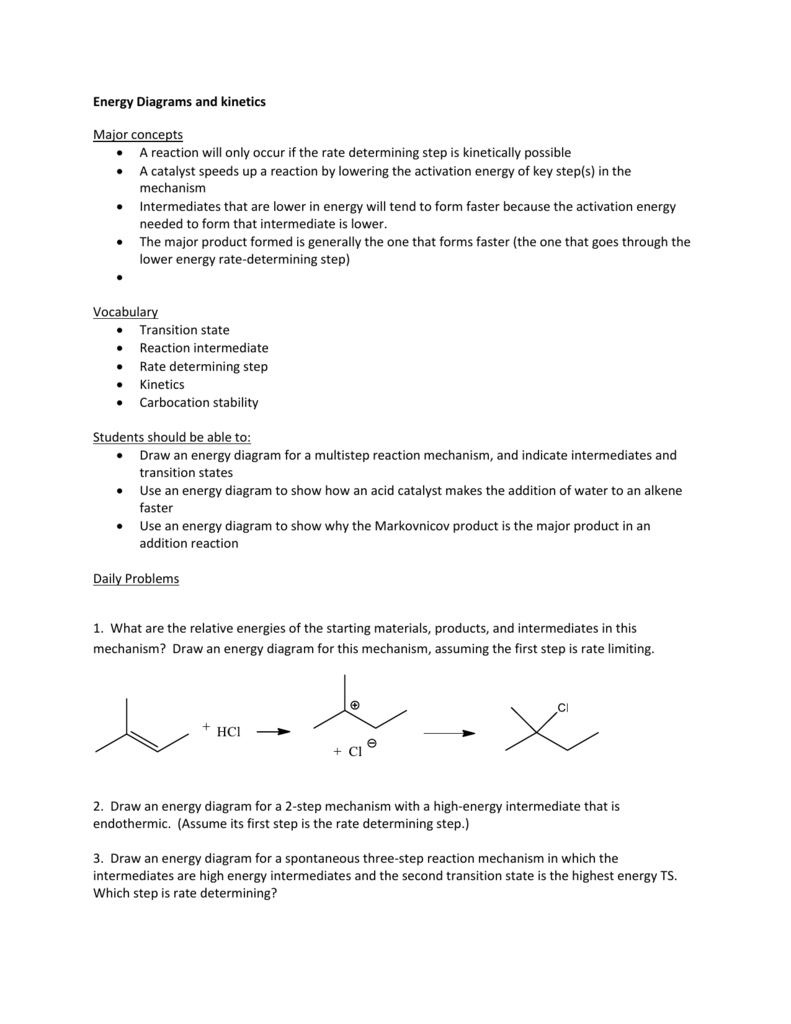
Where are the intermediates and transition states in this diagram?. Chemistry questions and answers. 2. How many transition states and intermediates would the reaction energy diagram have for the reaction shown below? сн. CH, CHE ОН CHE CI + H-CI o -- 10 C step 3 step 1 step 2 + co D A B A) Three transition states and three intermediates B) Two transition states and two intermediates C) Three transition ... Use the energy diagram for the reaction A D to answer the questions. How many transition states are there in the reaction? How many intermediates are there in the reaction? Which step of the reaction is the fastest? Which step of the reaction has the smallest rate constant? Assume the frequency factor (?) is the same for each elementary reaction. Problem: Energy profiles for the uncatalyzed and bromide-catalyzed decomposition of H2O2.Where are the intermediates and transition states in this diagram? FREE Expert Solution We're being asked to label the given energy diagram . Where are the intermediates and transition states in this diagram? Drag the appropriate labels to their respective targets. Note: not all targets will be used. Question: Where are the intermediates and transition states in this diagram? Drag the appropriate labels to their respective targets.
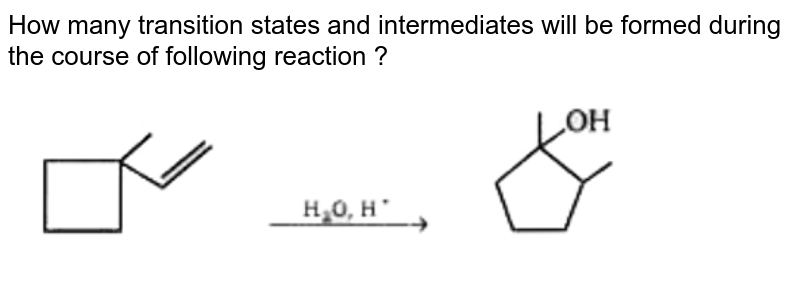
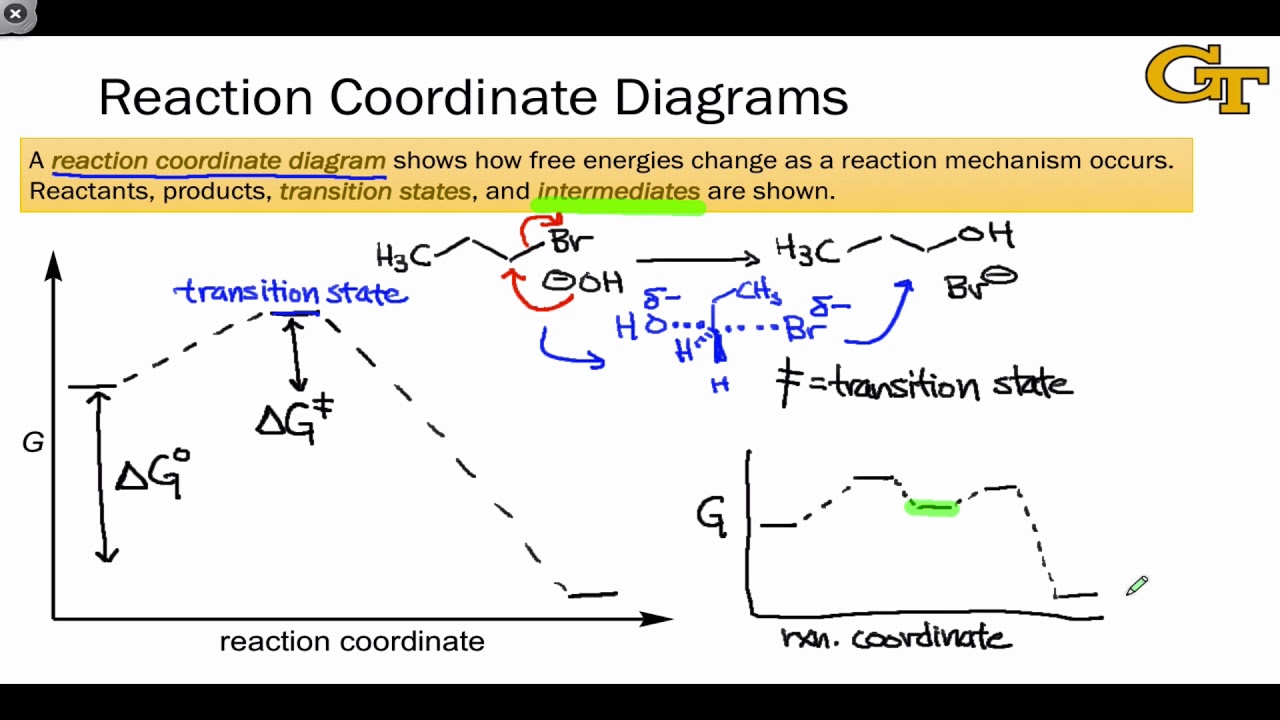
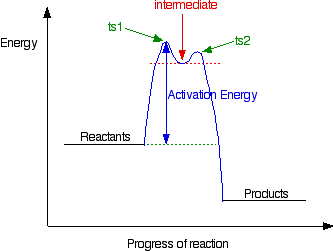
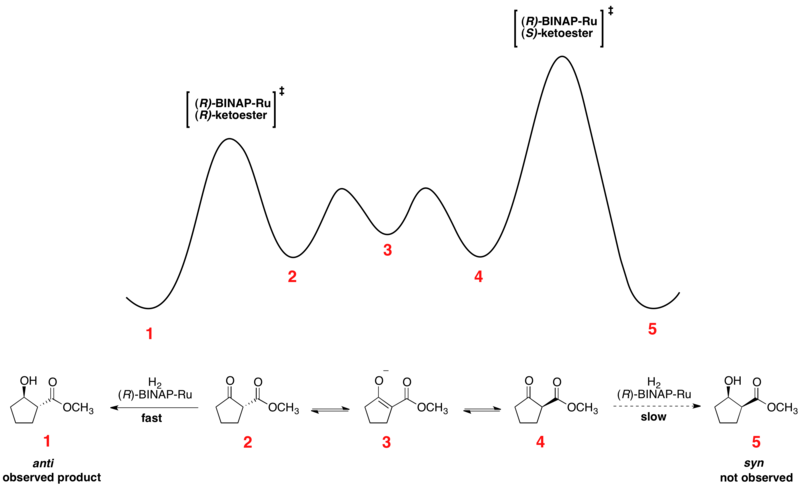
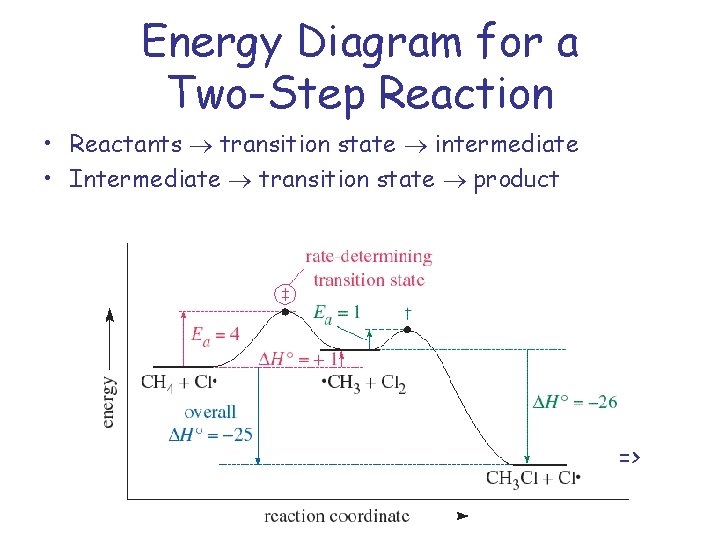
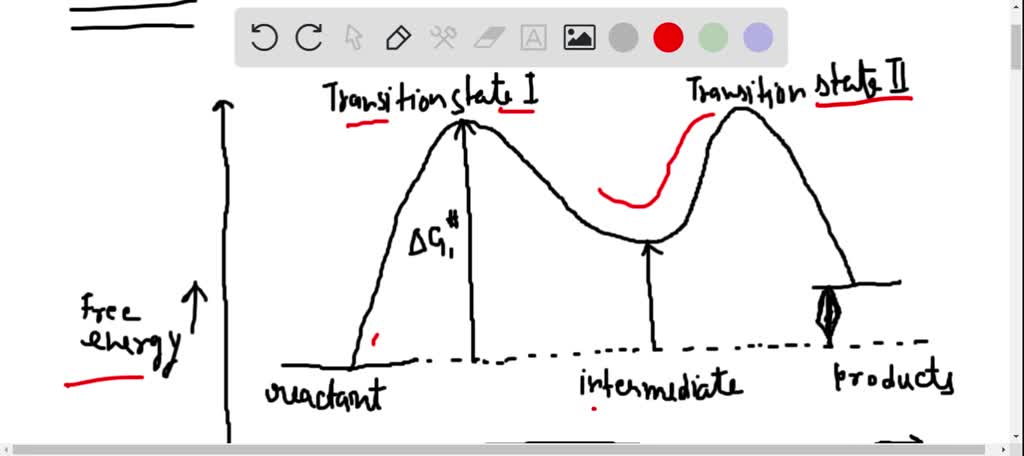
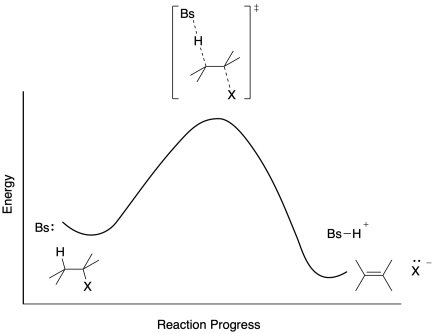
Energy diagrams are used to represent the change in energy for the molecules involved in a chemical reaction. The free energy is measured along the y- axis, and the reaction coordinate is plotted on the x- axis. The reaction coordinate indicates the progress of the conversion of reactants to products. Peaks on the energy diagram represent the transition states, whereas the valleys represent the reactive intermediates. and activation energy. Shown in Figure 5.2 is an energy diagram for the conversion of reac-tants to products in two steps. A reaction intermediate corresponds to an energy minimum between two transition states, in this case an intermediate between transition states 1 and 2. A diagram showing the relative standard Gibbs energies of reactants, transition states, reaction intermediates and products, in the same sequence as they occur in a chemical reaction. These points are often connected by a smooth curve (a 'Gibbs energy profile', commonly still referred to as a 'free energy profile') but experimental observation ... The second diagram where the bonds are half-made and half-broken is called the transition state, and it is at this point that the energy of the system is at its maximum.This is what is at the top of the activation energy barrier. But the transition state is entirely unstable.
Whereas, transition state is just the state before formation of new molecule (involves breaking of bonds of reactants and formation of new ones) An intermediate differs from a transition state in that the intermediate has a discrete lifetime (be it a few nanoseconds or many days), whereas a transition state lasts for just one bond vibration cycle. Download “Potential Energy Diagrams: Transition States and Intermediates” ATOOCV1-3-7-Potential-Energy-Diagrams-Transition-States-and-Intermediates.pdf – Downloaded 13 times – 885 KB Share this article/info with your classmates/friends and help them to succeed in their exams. A state diagram consists of states, transitions, events, and activities. You use state diagrams to illustrate the dynamic view of a system. They are especially important in modeling the behavior of an interface, class, or collaboration. State diagrams emphasize the event-ordered behavior of an object, which is especially useful in modeling ... Transition State. The transition state is the higher energy point between two minima along the reaction coordinate. Each step in a mechanism will have a transition state. The transition states are often very difficult to identify and during a reaction the molecules exist in this state for essentially zero time.
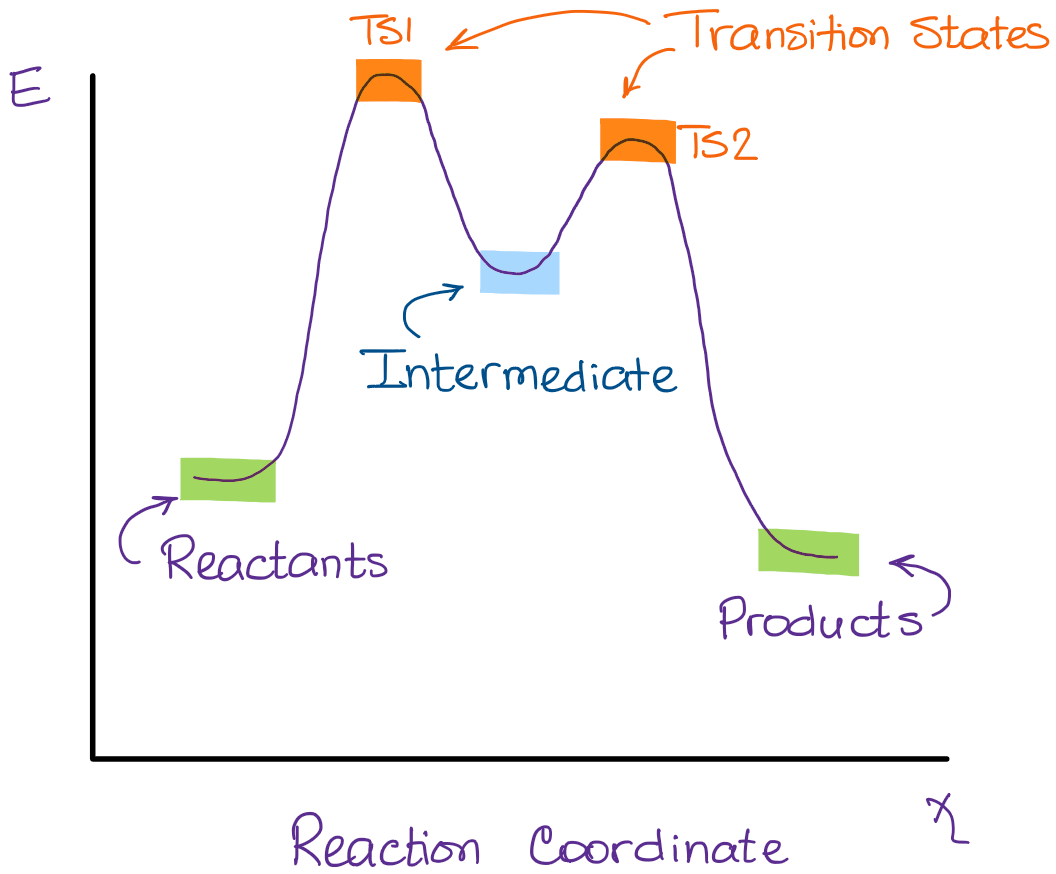
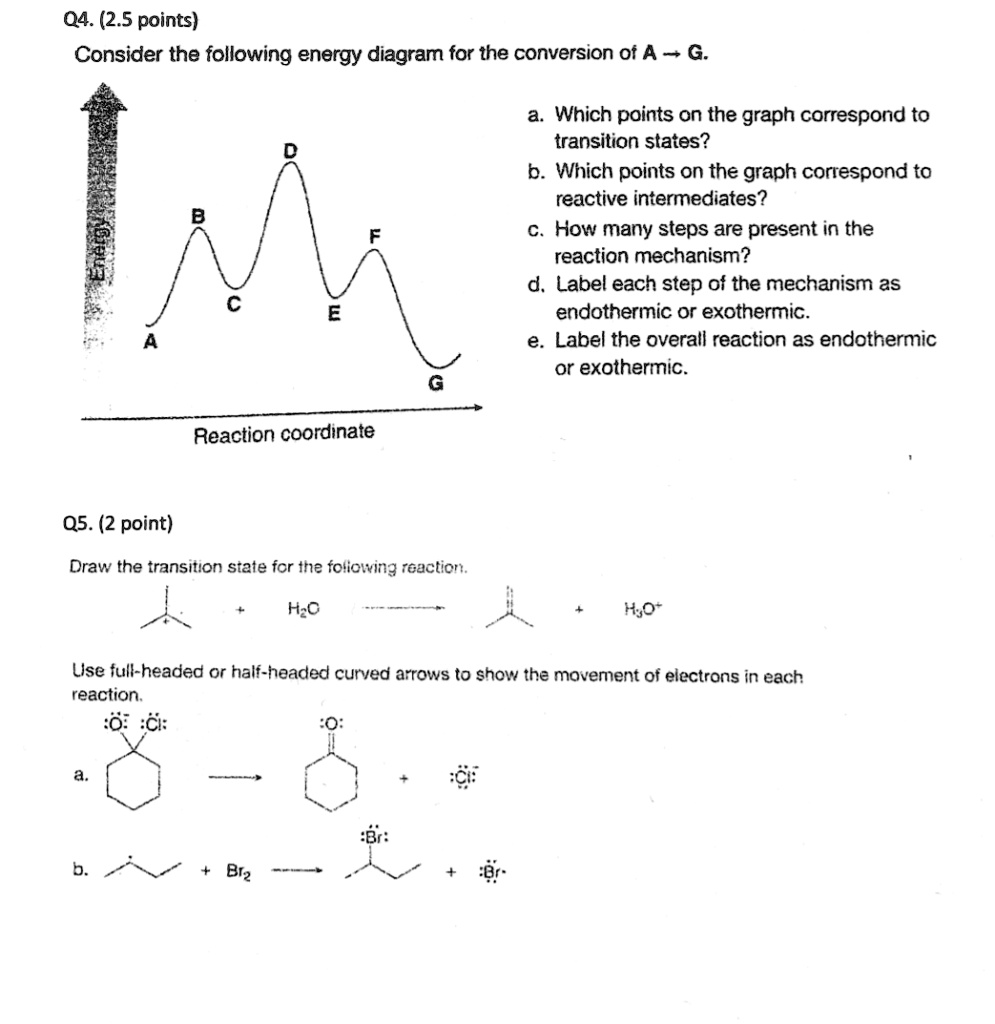
A) The transition states are located at energy minima. B) Each step is characterized by its own value of DH° and Ea. C) The rate-determining step has the lower energy transition state. D) The reactive intermediate is located at an energy maximum.
Energy Diagrams 6 • Transition state ‡: - An unstable species of maximum energy formed during the course of a reaction. - A maximum on an energy diagram. • Activation Energy, ∆G‡: The difference in Gibbs free energy between reactants and a transition state. - If ∆G‡ is large, few collisions occur with sufficient
This is part 3 of a four part series in the Energy Diagram Module. Stay tuned for Part 4! Click on the following links to see earlier parts: Part 1. Part 2. Sometimes reactions are more complex than simply a transition state (Graph 3), which would represent a single step in the reaction mechanism.
Reaction coordinate diagrams show the energies of transition states, intermediates, reactants and products Reaction Coordinate G transition state reactant product intermediate intermediateany chemical structure that lasts longer than the time of a typical bond vibration, 10-13 to 10-14 s rate-determining step (rds) the step of the reaction ...
This review study involves explanation about intermediate. any reacting species which is no longer a transition state. KEYWORDS: Reaction, Energy , Rate, Cation INTRODUCTION The easiest way to understand the difference between a transition state and an intermediate is to use what is commonly called a reaction (energy) diagram, like the one below.
Potential energy. Use the energy diagram for the reaction A → D to answer the questions. How many transition states are there in the reaction? transition states: B How many intermediates are there in the reaction? A D Reaction progress intermediates: Which step of the reaction is the fastest? Potential energy.
Energy Diagrams # Transition States vs. Intermediates! • A transition state occurs at an energy maxima. ! "The one on the left shows a Br- bond breaking and a Cl- bond forming." • Transition states cannot be isolated or directly observed.! • What might explain why transition states are so unstable?! Energy Diagrams # Transition States!
1. The Basic Equation. According to transition state theory, the rates of reactions are given by the following equation: where k is the rate constant for a given reaction, k is Boltzmann's constant, T is the absolute temperature, h is Planck's constant, and DG* is the free energy difference between the transition state and the reactants, i ...
Potential energy Diagram of Reactions with Reactant, Product, Transition State, and Intermediates The chemical species that are formed somewhere during the course of a chemical reaction are called as reaction intermediates. Unlike transition states, these are actual molecules that are short-lived and unstable.
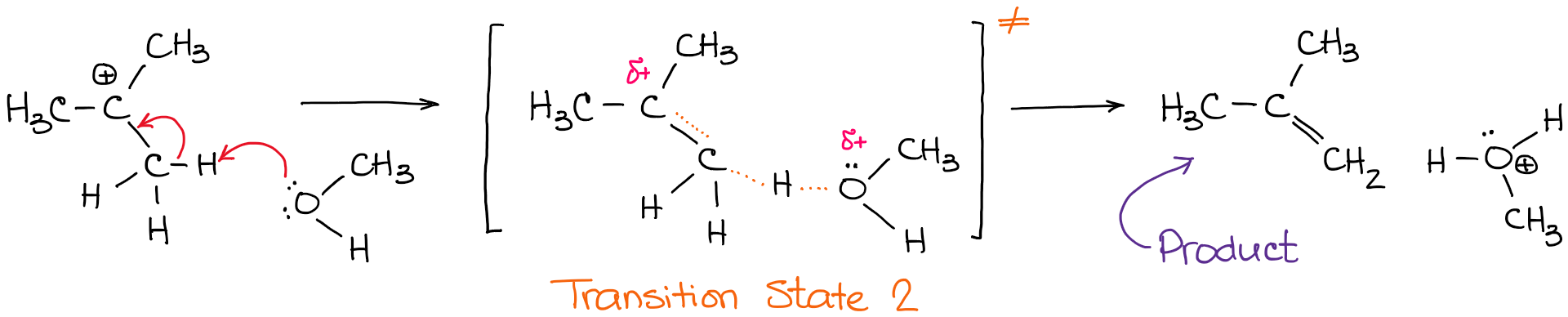
This particular intermediate configuration of atoms or molecules with the maximum value of potential energy is called activated complex and the state is defined as transition state. [Image will be Uploaded Soon] Now the diagram above shows the transition state of a chemical reaction taking place.
Transcribed text From Image: Where are the intermediates and transition states in this diagram? Drag the appropriate labels to their respective targets. Drag the appropriate labels to their respective targets.
here for the reaction. Coordinate diagram. Put two step verification in which the first step, Andrea Janek, the second step is extra, Janek. And the order reaction is and a sonic labels are re intense. Products, intermediates and chronic transition is start stairs. So this is the reaction co ordination diagram in which this is for free energy, the bikes and X axis for the the reaction coordinates.
Subject : ChemistryPaper : Organic Chemistry-IIModule : Intermediates, Transition states, Potential energy diagrams (CHE)Content writer :
The reaction diagram above has 2 intermediates and 3 transition states, so it is a 3-step reaction. Finally, the last question you can expect is a question about the shape or a nature of the transition state itself. We know that the transition state is something in-between the reagents and products/intermediate.
May 30, 2020 — ... Reaction Energy Diagram to discuss transition states, Ea, intermediates & rate determining step; draw the transition state of a reaction.
Transition States vs. Intermediates! A transition state is an unstable species! (it has no measurable lifetime)! Reaction Coordinate! An intermediate has a measureable lifetime! (it can be isolated in theory)! The energy of activation refers to the energy difference between the starting material and the transition state along the reaction ...
Draw a graph showing the reaction pathway for an overall exothermic reaction with two intermediates that are produced at different rates. On your graph indicate the reactants, products, intermediates, transition states, and activation energies. Learn this topic by watching Energy Diagram Concept Videos.























Komentar
Posting Komentar