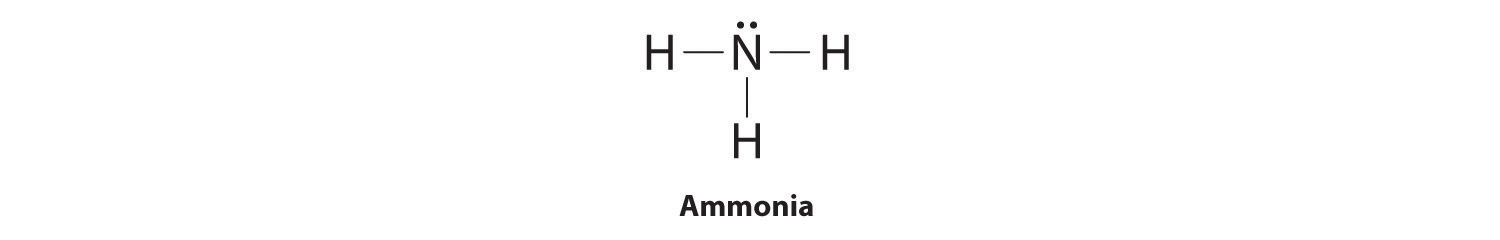
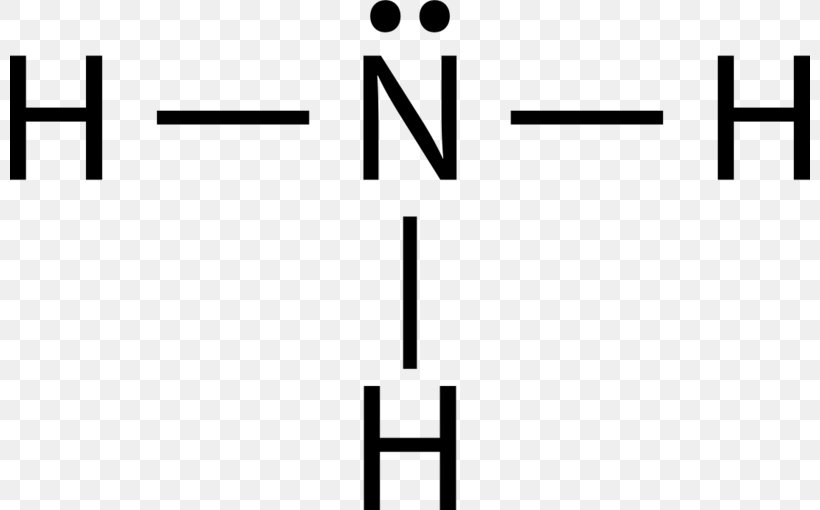
39 electron dot diagram for ammonia
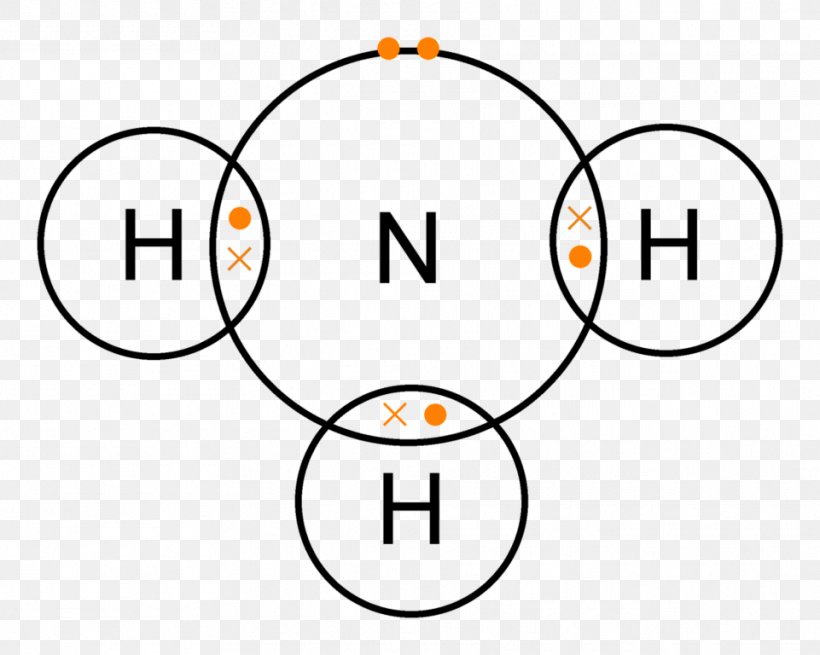
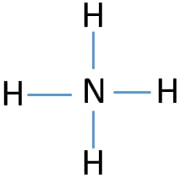
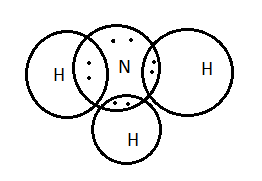
Lewis structure - ammonia - YouTube A simple method for drawing the Lewis structure for ammonia. Electron Dot Diagram For Nh3 - schematron.org Nitrogen goes in the centre. Lewis dot structure of ammonia. Alternatively a dot method can be used to draw the lewis structure of NH3. Calculate the total valence electrons in NH3 molecule. N=5,H=1x3=3 Total=8 Put Nitrogen in the center and three hydrogen atoms on the sides. We're going to do the Lewis structure for NH3: ammonia or Nitrogen trihydride.
Solved Writing the Lewis structures for a molecule ... - Chegg Chemistry. Chemistry questions and answers. Writing the Lewis structures for a molecule with resonance Draw the Lewis structure for the ammonia (NH,) molecule. Be sure to include all resonance structures that satisfy the octet rule. Explanation Check o 2010.

Electron dot diagram for ammonia
Electron Dot Diagram Of Ammonium Ion - schematron.org Jan 05, 2019 · and put a dot and a cross in each overlapping section (there should be three dots and three crosses in the whole diagram Actually the formula for an ammonia ion is NH4+ Due to dative bonding the 4th hydrogen loses an electron making it a positive ion and allowing it to form a dative bond with the Nitrogen. Meaning the nitrogen. Lewis Structure for NH3 (Ammonia) - UMD Drawing the Lewis Structure for NH 3 ( Ammmonia) Ammonia (NH 3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer. It also is a good example of a molecule with a trigonal prymidal molecular geometry. There are 8 valence electrons available for the Lewis structure for NH 3. Draw the electron dot structure of Ammonium ion [N = 7, H = 1] Draw the electron dot structure of Ammonium ion [N = 7, H = 1] Solve Study Textbooks Guides. >>. Class 11. >> Chemistry. >> Chemical Bonding and Molecular Structure. >> Basics of Chemical Bonding. >> Draw the electron dot structure of Ammon.
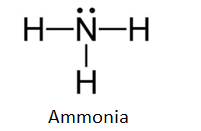
Electron dot diagram for ammonia. What is the electron dot structure of ammonia? Ammonia is made out of one nitrogen and three hydrogen atoms. Its structure is tetrahedral. Ammonia is used in nitric acid production, as a fertilizer, and a cleaning solution. NH3, normally found as a gas, it is caustic and harmful in longterm exposure. NH4+ (Ammonium ion) Lewis Structure NH 4 + lewis structure. You see, there are four hydrogen atoms around nitrogen atom. Therefore, nitrogen atom is the center atom. Also, there is a +1 charge on nitrogen atom. Steps of drawing lewis structure of NH 4 + There are several steps to draw the lewis structure of NH 4 +. But, These steps are explained in detail in this tutorial. NH3 Lewis Structure - How to Draw the Dot Structure for ... A step-by-step explanation of how to draw the NH3 Lewis Dot Structure (Ammonia).For the NH3 structure use the periodic table to find the total number of vale... What Is The Lewis Dot Structure For Ammonia ... Is NH3 a Lewis structure? In the lewis structure of ammonia (NH3), there are three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH3 can be drawn by starting from valence electrons of nitrogen and hydrogen atoms in several steps. ... After drawing the lewis structure of NH3, you can decide shape of the NH3 molecule.
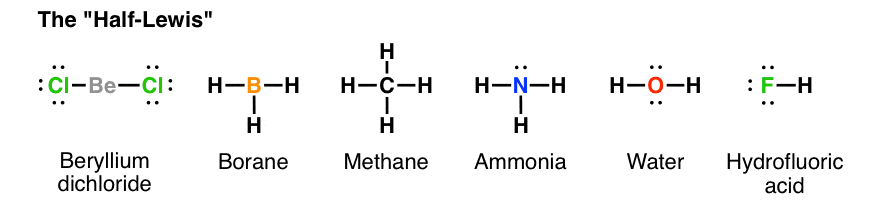
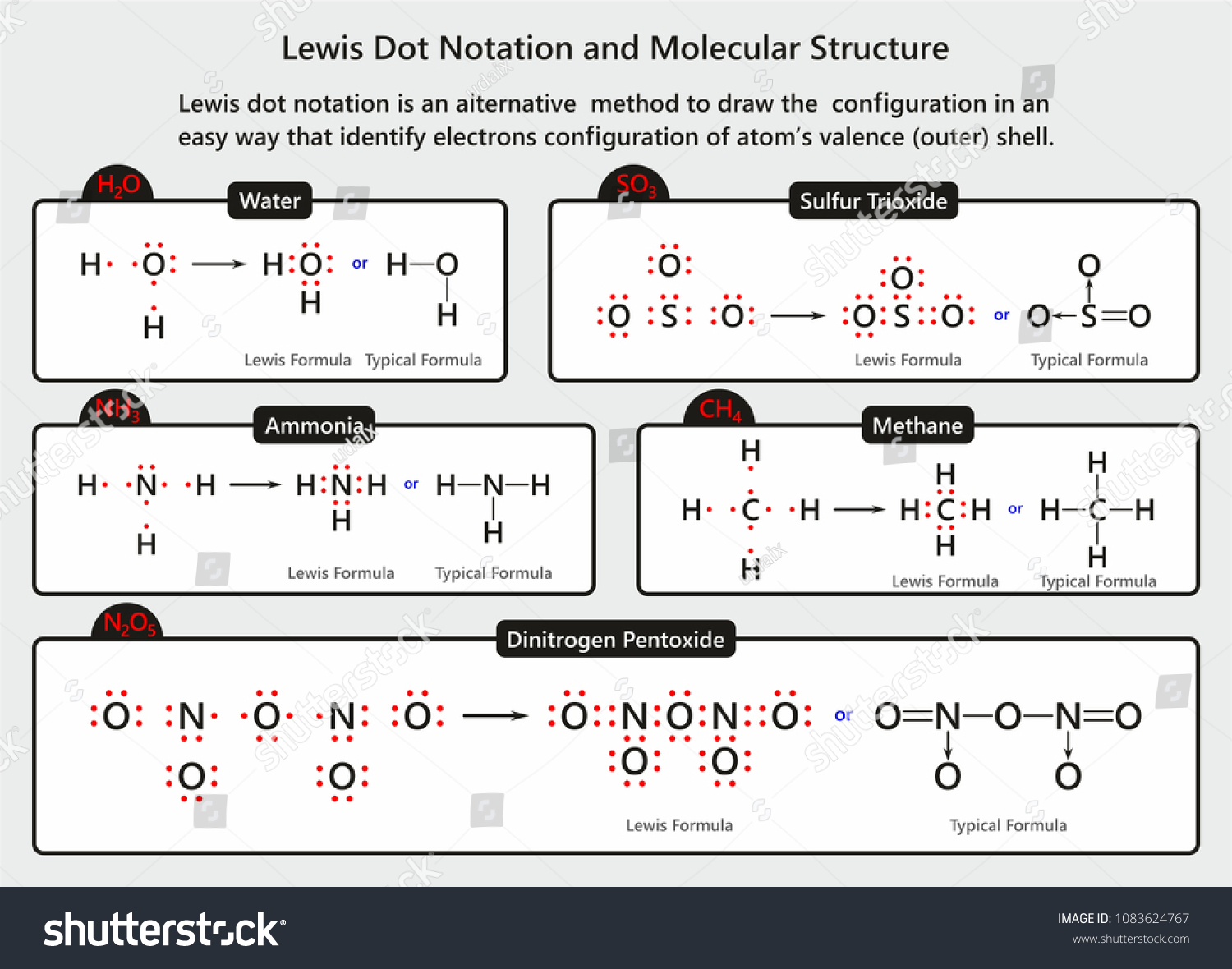
Electron Dot Diagrams | Chemistry for Non-Majors Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ... Solved Draw the Lewis electron dot diagram for ammonia ... Which of the following is true regarding the Lewis diagram for ammoni a? (2 points) a. Ammonia contains three covalent bonds, one to each hydrogen atom, and the nitrogen atom has no lone pairs of electrons. b. Question: Draw the Lewis electron dot diagram for ammonia (NH3) on a sheet of scrap paper. You may consult the periodic table on Zoom to ... Ammonia (NH3) Lewis Structure - Steps of Drawing In the lewis structure of ammonia (NH 3), there are three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH 3 can be drawn by starting from valence electrons of nitrogen and hydrogen atoms in several steps. Each step of drawing the lewis structure of NH 3 is explained in detail in this tutorial. What is the Lewis dot diagram for nh3? - FindAnyAnswer.com Explanation: The Lewis structure of ammonia, NH3 , would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the atom. This is the reason why ammonia acts as a Lewis base, as it can donate those electrons.
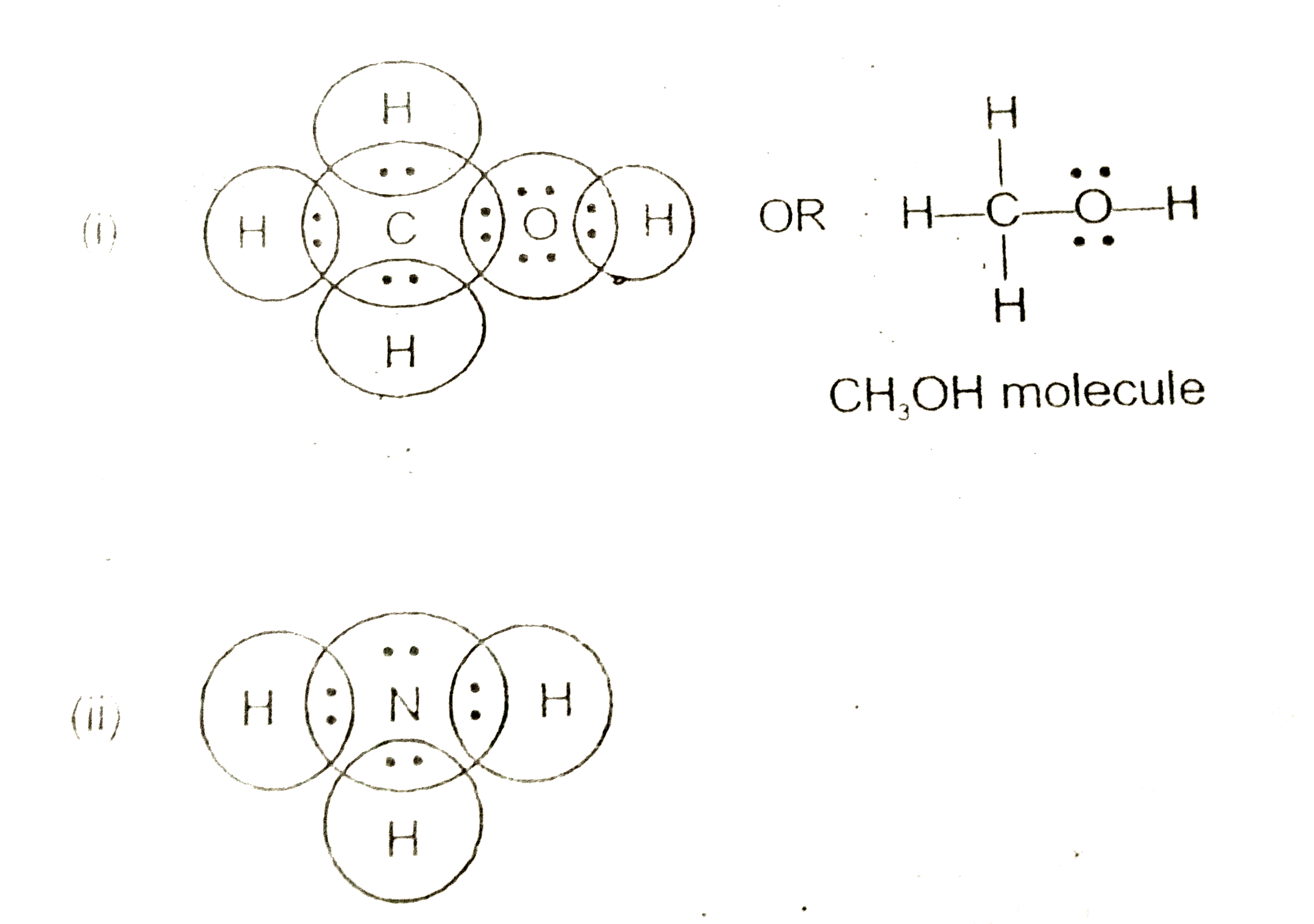
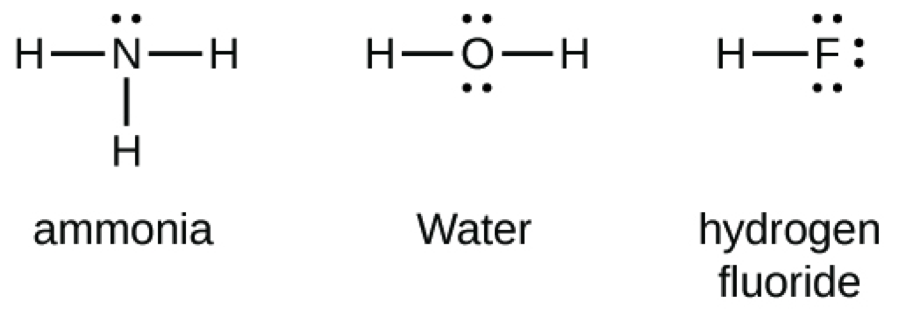
Lewis Structures (electron dot diagrams) Chemistry Tutorial Lewis Structure (electron dot diagram) for ammonia OR . Note that there are 3 covalent bonds (3 bonding pairs of electrons) in total, and that there is a lone pair (non-bonding pair) of electrons on the nitrogen atom. In the Valence Structure for ammonia, the bonding pairs of electrons, which may or may not be circled in the Lewis structure ... Lewis Electron Dot Structures - Detailed Explanation with ... Lewis structures, also known as electron-dot or electron-dot diagrams, are diagrams showing the bonding between a molecule's atoms and the lone pairs of electrons that may occur in the molecule. What is the Lewis structure of ammonia? Ammonia has the NH3 equation. It is extremely water-soluble because it is a polar material. What is the electron dot structure for nh3? The electron-dot structure of NH3 places one pair of nonbonding electrons in the valence shell of the nitrogen atom. This means that there are three bonded atoms and one lone pair for a coordination number of four around the nitrogen, the same as occurs in H2O. The Lewis dot structure for ammonia, NH3. Additionally, what is the shape of nh3 ... Lewis Dot Diagram Of Nh3 Lewis Dot Diagram Of Nh3 The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Electron Dot Structure of NH3 by Jeff Bradbury - February 17, - Lewis Electron Dot Structure for ammonia molecule NH3. by crator-avatar Jeff Bradbury 2.
Electron Dot Structure of NH3 | Chemistry, Science | ShowMe Electron Dot Structure of NH3 by Jeff Bradbury - February 17, 2012 - Lewis Electron Dot Structure for ammonia molecule NH3
What is the Lewis dot structure for ammonia? – Colors-NewYork.com Can you draw the electron dot structure of ammonia? Out of which 3 electrons of nitrogen form a covalent bond with hydrogen, with sharing of one electron of nitrogen and one electron of hydrogen. There are three such bonds. So, two electrons are left as a lone pair. This is the structure of ammonia or NH3. What is the use of Lewis dot structure?
NH3 Lewis Structure, Molecular Geometry, Hybridization ... Ammonia or NH3 has a total of 8 valence electrons. NH3 Lewis Structure. The Lewis structure of a molecule helps understand the electron geometry, molecular geometry, polarity and other such properties with ease. It is a pictorial representation of the arrangement of valence electrons around the individual atoms in the molecule.
NH3 Lewis Structure, Geometry, and Hybridization ... In addition to this, ammonia is considered corrosive as well as hazardous if stored in significantly larger quantities. The lewis structure that is also called an electron dot structure, is mainly a pictorial representation of the valence electrons present in an atom. The diagram is drawn using dots around the symbol of an atom, mostly in pairs.
What is Lewis structure of ammonia? - MSI The ammonia molecule has a trigonal pyramidal shape as predicted by the valence shell electron pair repulsion theory (VSEPR theory) with an experimentally determined bond angle of 106.7°. The central nitrogen atom has five outer electrons with an additional electron from each hydrogen atom.
41 draw an electron dot diagram for ammonia - Wiring ... After counting the valence electrons, we have a total of 9 [5 from nitrogen + 4 (1 from each hydrogen)] = 9. and put a dot and a cross in each overlapping section (there should be three dots and three crosses in the whole diagram Actually the formula for an ammonia ion is NH4+ Due to dative bonding the 4th hydrogen loses an electron making it a ...
NH3 Lewis Structure (Ammonia) - YouTube Hey Guys. Today in this video we are going to look at the Lewis Structure of Ammonia. It has a chemical formula of NH3 and is a pungent-smelling molecule tha...
Lewis Structure Of Nh3 - ViralListClub.com Lewis Dot Diagram Of Nh3 The Lewis structure of ammonia NH3 would be three hydrogen atoms bonded to a nitrogen atom in the middle with a lone pair of electrons. Ammonia NH3 or H3N CID 222 - structure chemical names physical and chemical properties classification patents literature biological activities safety.
Draw the electron dot stucture of ammonia molecule. - Toppr Solution. Step 1: Find valence e- for all atoms. Add them together. N-5 H-1x3=3 Total=8. Step2: Find octet e- for each atom and add them together. Step3: Gives you bonding e-. Subtract step 1 total from step 2 14-8=6e-. Step 4: Find number of bonds by diving the number in step 3 by 2 (because each bond is made of 2 e-) 6e-/2= 3 bond pairs. Step ...
38 ammonia electron dot diagram - Wiring Diagrams Manual Electron Dot Diagram Of Ammonium Ion - schematron.org and put a dot and a cross in each overlapping section (there should be three dots and three crosses in the whole diagram Actually the formula for an ammonia ion is NH4+ Due to dative bonding the 4th hydrogen loses an electron making it a positive ion and allowing it to form a dative bond with the Nitrogen.
Draw the electron dot structure of Ammonium ion [N = 7, H = 1] Draw the electron dot structure of Ammonium ion [N = 7, H = 1] Solve Study Textbooks Guides. >>. Class 11. >> Chemistry. >> Chemical Bonding and Molecular Structure. >> Basics of Chemical Bonding. >> Draw the electron dot structure of Ammon.
Lewis Structure for NH3 (Ammonia) - UMD Drawing the Lewis Structure for NH 3 ( Ammmonia) Ammonia (NH 3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer. It also is a good example of a molecule with a trigonal prymidal molecular geometry. There are 8 valence electrons available for the Lewis structure for NH 3.
Electron Dot Diagram Of Ammonium Ion - schematron.org Jan 05, 2019 · and put a dot and a cross in each overlapping section (there should be three dots and three crosses in the whole diagram Actually the formula for an ammonia ion is NH4+ Due to dative bonding the 4th hydrogen loses an electron making it a positive ion and allowing it to form a dative bond with the Nitrogen. Meaning the nitrogen.








![Draw the electron dot structure of Ammonium ion [N = 7, H = 1]](https://d1hj4to4g9ba46.cloudfront.net/questions/1890011_1909650_ans_86e4b88529e541639e84039753e5b955.png)


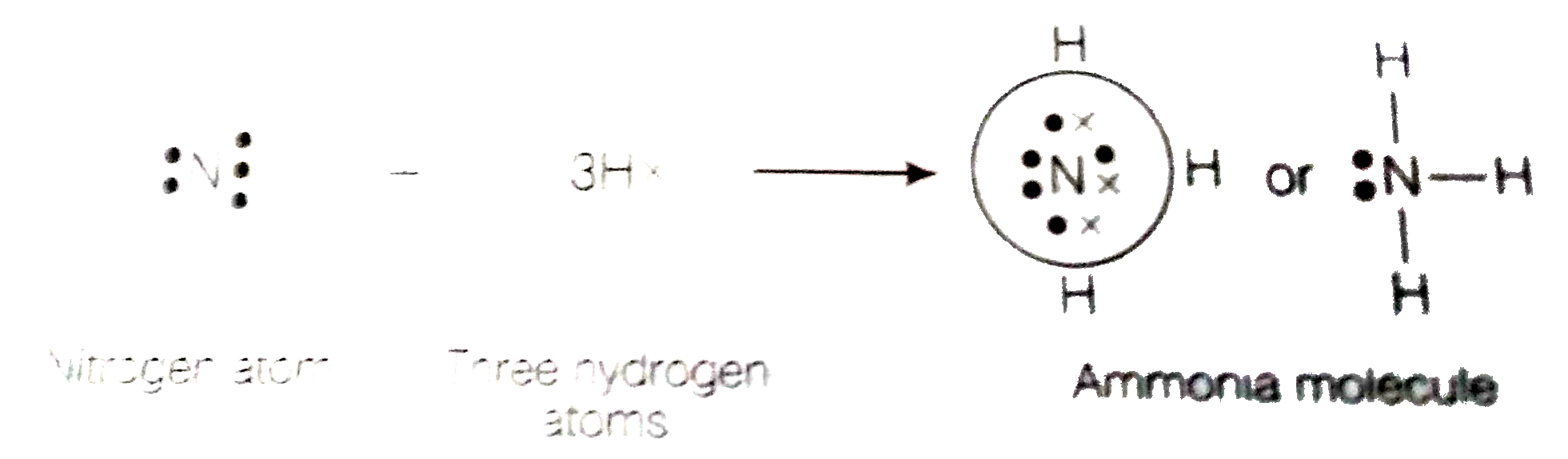








Komentar
Posting Komentar