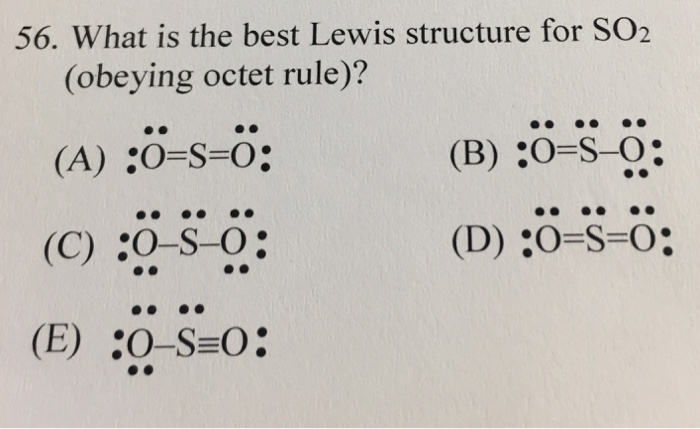
40 lewis dot diagram for so2
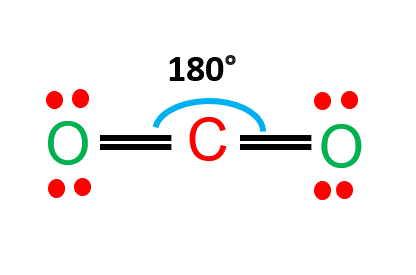
CO2 (Carbon Dioxide) Lewis Dot Structure - Science Trends The Lewis Dot Structure for carbon dioxide can be represented like this:. o=C=o. But what exactly does this mean? What is a Lewis Dot Structure, and what do the symbols in carbon dioxide's structure represent?Let's go over the Lewis structure and find out how to interpret this representation of carbon dioxide. So2 Lewis Dot Diagram - schematron.org The Lewis dot structure of SO2, or sulfur dioxide, has a central atom of sulfur that violates the octet rule. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms. Sulfur has valence electrons in the 3rd energy level, allowing access . Drawing the Lewis Structure for SO 2.
Sulfor dioxide: Lewis dot structure for SO2 (video) | Khan ... The dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a double bond to an oxygen on the right, and two lone pairs of electrons on that oxygen. And then we have a lone pair of electrons on our sulfur.
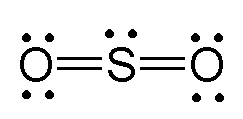
Lewis dot diagram for so2
Solved lewis dot structure for SO2? | Chegg.com This problem has been solved! lewis dot structure for SO2? Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Lewis Structure of SO2 (sulfur dioxide) - YouTube How to draw the Lewis Structure of SO2 - with explanationCheck me out: NO2 (Nitrogen Dioxide) Lewis Dot Structure Nitrogen Dioxide (NO 2) is a covalent compound that is composed of a central nitrogen atom single bonded to an oxygen atom and a double bond with another oxygen atom. At room temperatures, nitrogen dioxide is a reddish-brown gas that has a density of 1.8 g/dm 3.It is slightly toxic to humans, on account of its tendency to react in the human body and produce reactive species of nitrogen and ...
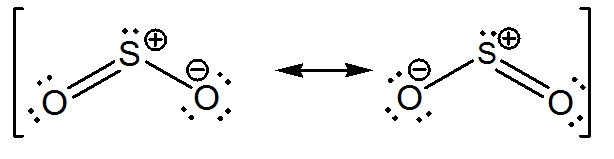
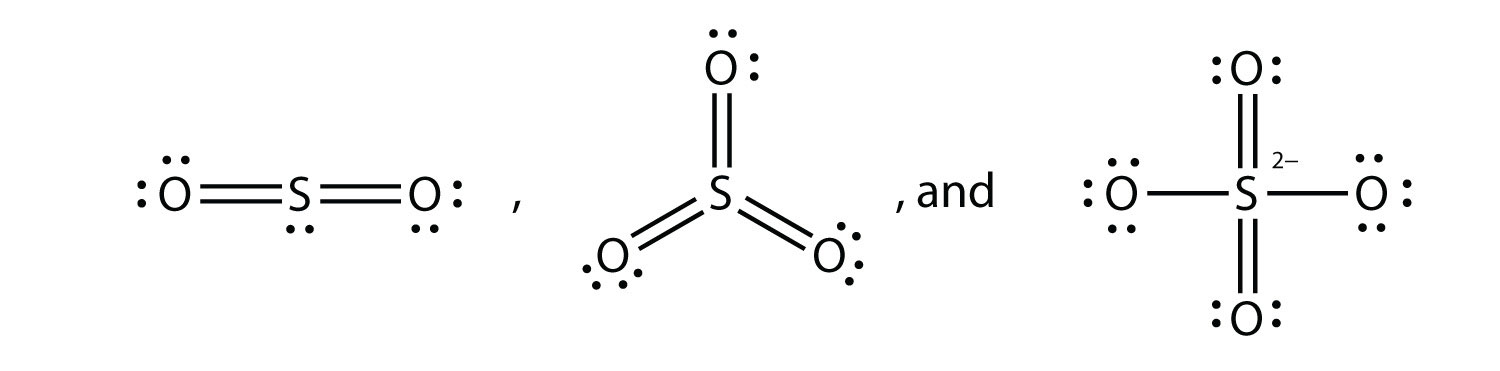
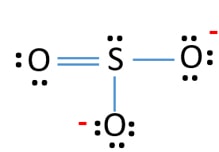
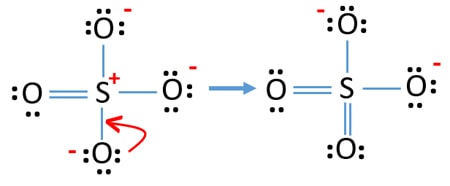
Lewis dot diagram for so2. SO2 Lewis Structure - Lewis Dot Structure | Chem Helps Lewis Structure For SO2 For some molecules and ions, a single Lewis structure is not sufficient. For example, SO2 Lewis Structure can be drawn as: Speaking for the SO2 Lewis Structure, one of the oxygen atoms is attached to the sulfur atom with a single bond and the other with a double bond. Single bonds should be longer than double bonds. How can you determine the dot diagram for SO2? - Quora 17 Aug 2016 — To draw the lewis structure for SO2, we have to find out the valence electrons of sulfur and oxygen first.We express valence electrons as dots in lewis dot ... Draw the Lewis dot diagram for the following | Chegg.com Draw the Lewis dot diagram for the following reaction: carbon plus oxygen makes carbon dioxide. Hint: first determine what kind of bond will be produced (ionic vs covalent) and how many of each atom you will need. Question: Draw the Lewis dot diagram for the following reaction: carbon plus oxygen makes carbon dioxide. Hint: first determine what ... Lewis Diagram For So2 - schematron.org Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond. The Lewis dot structure of SO2, or sulfur dioxide, has a central atom of sulfur that violates the octet rule. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms.
SO2 Lewis Structure - How to Draw the Lewis Structure for ... A step-by-step explanation of how to draw the SO2 Lewis Structure (Sulfur Dioxide) Note: From an experimental view (using x-ray crystallography or someth... How to draw SO2 Lewis Structure? - Science Education and ... To sketch the SO2 Lewis structure by following these instructions: Step-1: SO2 Lewis dot Structure by counting valence electrons on the sulfur atom. Step-2: Lewis Structure of SO2 for counting valence electrons around the terminal oxygen atoms. Step-3: Lewis dot Structure for SO2 generated from step-1 and step-2. What does the Lewis dot structure look like for sulfur ... bohr model for so2 What does the Lewis dot structure look like for sulfur? The electron configuration for sulfur is 1s2 2s2 2p6 3s2 3p4. This means there are six valence electrons. two double dots... Lewis Structure of SO2 [duplicate] - Chemistry Stack ... 8 Dec 2017 — The reason is that the octet rule is observed this way (no hybridization of d-orbitals for main group chemistry necessary or possible) as well ...
NO2 (Nitrogen Dioxide) Lewis Dot Structure | Science Trends NO2 (Nitrogen Dioxide) Lewis Dot Structure. Nitrogen Dioxide (NO 2) is a covalent compound that is composed of a central nitrogen atom single bonded to an oxygen atom and a double bond with another oxygen atom. At room temperatures, nitrogen dioxide is a reddish-brown gas that has a density of 1.8 g/dm 3. SO2 Lewis dot structure (diagram) | PopScreen The Foolproof way to Draw Lewis Structures; Drawing Lewis Structures; Carbonate ion (CO3) Lewis Structure; Methane Lewis dot structure (diagram) 8.2 Lewis Dot Structures; Valence Electrons with Dots; 14 2 3 Lewis, hybridization (sp3,sp2,sp) , shapes and angles IB chemistry HL; AP Video 8.2 - Drawing LS, Exceptions, & Resonance; Covalent Bonding ... Dot Diagram For So2 - Wiring Diagrams Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond. The Sulfur Dioxide which is also known as Sulphur Dioxide is the entity of a bond between Sulfur and Oxygen atoms. It is known as a formula written as SO2. SO2(Sulfur Dioxide) Lewis Structure, Hybridization ... SO2 Lewis Structure. Now that we've calculated the number of valence electrons available to us, we move on towards building up the Lewis structure for SO 2. Sulfur is the least electronegative atom in the compound and will act as the central atom. The two oxygen atoms are arranged adjacent to the central sulfur atom.
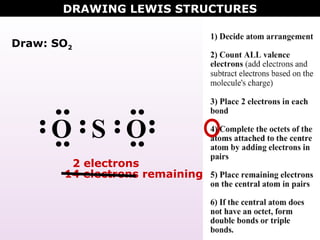
(15) Lewis Diagrams (15) Lewis Diagrams Obj. 15. From the name of a molecular chemical, determine the Lewis (electron dot) diagram for it.. In these cases, you know that you are dealing with nonmetal atoms bonded together with covalent bonding and that (some of) the valence electrons of the atoms are shared between the atoms.
CO2 Lewis Structure (2021 UPDATED) All You Need To Know CO2 or carbon dioxide is considered as acid or can even be called Lewis Acid. The resonance structure accepts lone pairs of electrons, but the three lone pairs of electrons are in the oxygen molecule. There are no lone pairs of electrons in CO 2. Although, when dissolved in water, CO 2 takes the form of carbonic acid using this formula.
SO2 Lewis Structure: How to Draw the Dot Structure for S02 ... Drawing the Lewis Structure for SO 2 Viewing Notes: The Lewis structure for SO 2 requires you to place more than 8 valence electrons on Sulfur (S). You might think you've got the correct Lewis structure for SO 2 at first. Remember, Sulfur is in Period 3 and can hold more than 8 valence electrons.
Answered: what is lewis dot structure of SO2. | bartleby Browse 5+ million homework and textbook solutions, concept explainers, videos and more. Search concepts or drop in your homework problem! Our library grows every minute-keep searching! Science Chemistry Q&A Library what is lewis dot structure of SO2.
Lewis Dot of Sulfur Dioxide SO2 - kentchemistry.com Lewis Dot of Sulfur Dioxide. SO 2. Back. 70 More Lewis Dot Structures. S does not follow the octet rule. It can hold more than 8 electrons. Sulfur having valence electrons in the 3rd energy level, would also have access to the 3d sublevel, thus allowing for more than 8 electrons. NOT IN THIS MOLECULE.
Draw Lewis electron dot structure of SO2 - Chemistry ... Draw Lewis electron dot structure of SO2 . Maharashtra State Board HSC Science (General) 11th. Textbook Solutions 8028. Important Solutions 18. Question Bank Solutions 5553. Concept Notes & Videos 418. Syllabus. Advertisement Remove all ads. Draw Lewis electron dot structure of SO2 - Chemistry ...
Lewis Structure SO2? - Answers This is the Lewis structure for SO2 - O:S:O. Note is is bent planar. Wiki User. ∙ 2011-09-13 11:15:45. This answer is:
What is the Lewis dot structure of so2? - AskingLot.com What is the Lewis dot structure of so2? SO2 Lewis structure. To create the Lewis structure of SO2, you need to arrange the eight valence electrons on the Sulphur. To design the best Lewis structure, you also need to calculate the formal charge of every atom too. You know that both the Sulphur and Oxygen has six valence electrons each.
What is the lewis structure for SO_2? | Socratic Here are the steps I follow when drawing a Lewis structure. > 1. Decide which is the central atom in the structure. That will normally be the least electronegative atom ("S"). 2. Draw a skeleton structure in which the other atoms are single-bonded to the central atom: "O-S-O". 3. Draw a trial structure by putting electron pairs around every atom until each gets an octet. In this editor, I will ...
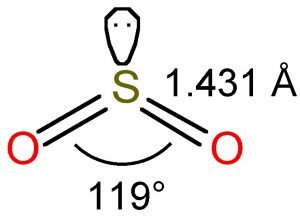
SO2 Lewis Structure| 4 Simple Steps - What's Insight SO2 Lewis Structure- Key Points. The electron geometry of SO 2 is formed in the shape of a trigonal planner. The three pairs of bonding electrons arranged in the plane at an angle of 120-degree. The sulfur's valence electron = 6; valence electrons of oxygen = 6 (There are 2 oxygen atoms in the compound)
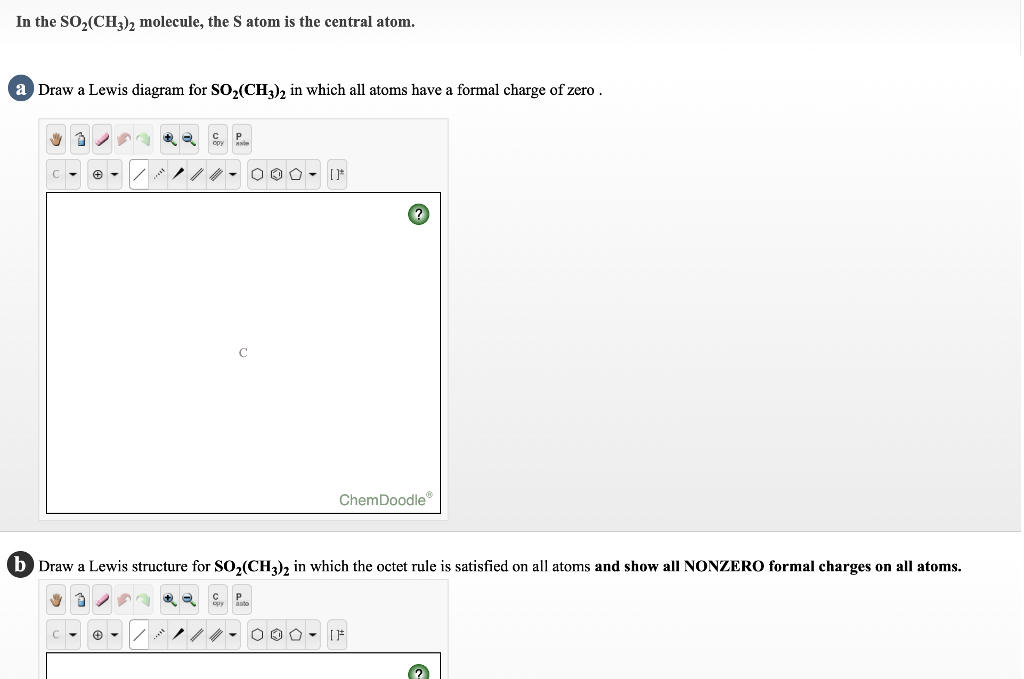
Draw a Lewis structure for SO2 in which al... | Clutch Prep Problem: Draw a Lewis structure for SO2 in which all atoms obey the octet rule. Show formal charges.Draw a Lewis structure for SO2 in which all atoms have a formal charge of zero. Explicitly showing the zero charges is optional. Do not consider ringed structures.
Sulfur dioxide (SO2) Lewis Structure, Hybridization Sulfur dioxide (SO 2) Lewis Structure, Hybridization. Sulfur dioxide molecule contains one sulfur atom and two oxygen atoms. We will construct the lewis structure of SO 2 molecule by following VSEPR theory rules and considering stability of intermediate structures. After obtaining the lewis structure of SO 2, we can determine the hybridization of atoms.
NO2 (Nitrogen Dioxide) Lewis Dot Structure Nitrogen Dioxide (NO 2) is a covalent compound that is composed of a central nitrogen atom single bonded to an oxygen atom and a double bond with another oxygen atom. At room temperatures, nitrogen dioxide is a reddish-brown gas that has a density of 1.8 g/dm 3.It is slightly toxic to humans, on account of its tendency to react in the human body and produce reactive species of nitrogen and ...
Lewis Structure of SO2 (sulfur dioxide) - YouTube How to draw the Lewis Structure of SO2 - with explanationCheck me out:
Solved lewis dot structure for SO2? | Chegg.com This problem has been solved! lewis dot structure for SO2? Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.


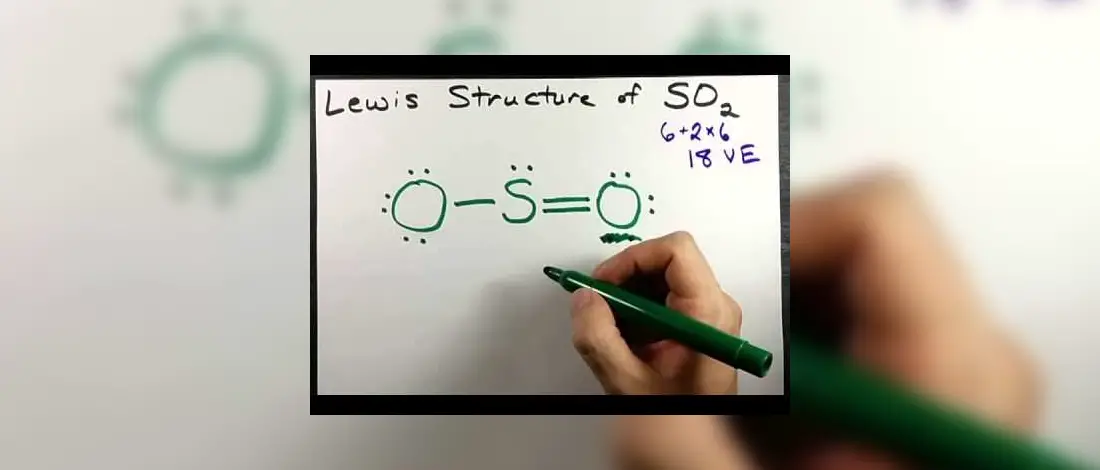























Komentar
Posting Komentar