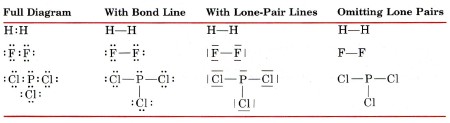
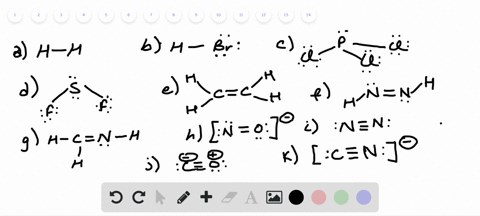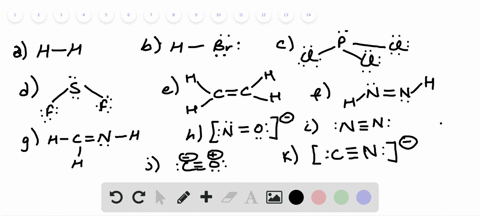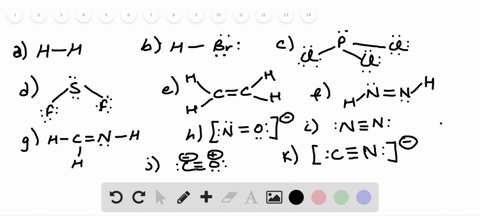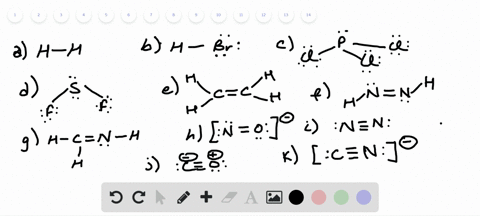
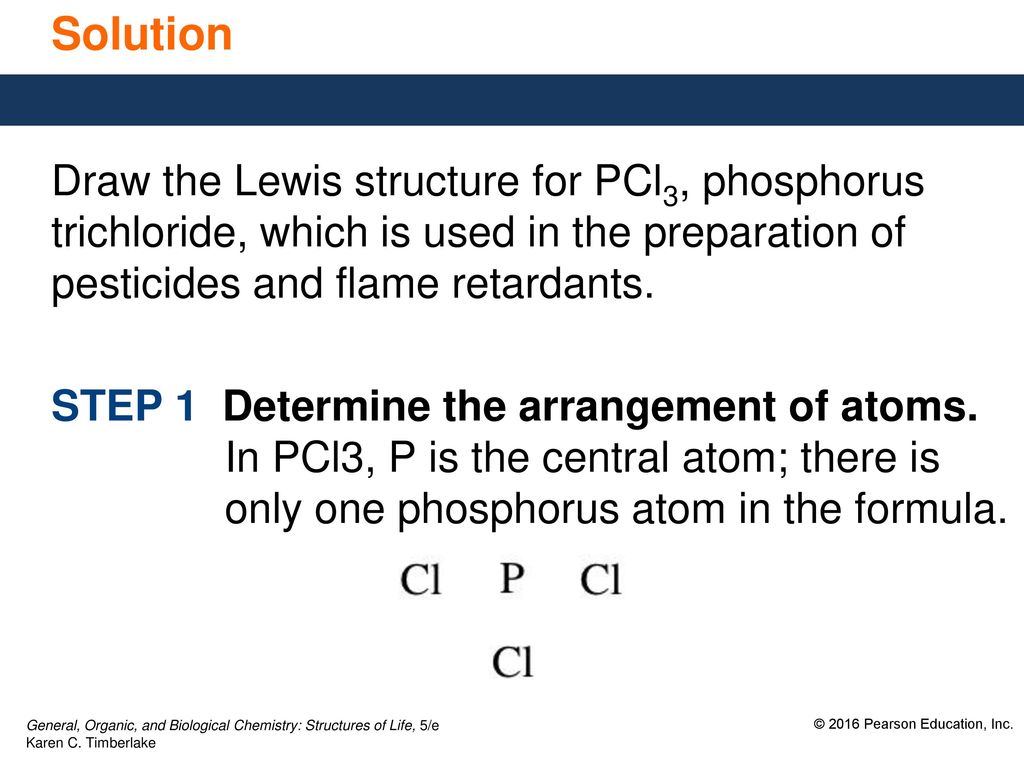
42 lewis dot diagram for pcl3
Nitrogen trichloride (NCl3) lewis dot structure, molecular ... Nitrogen trichloride (NCl3) lewis dot structure, molecular geometry, polar or non-polar, hybridization. Nitrogen trichloride is a very explosive substance that appears like an oily liquid with the chemical formula NCl3. It smells similar to chlorine. It has a dipole moment of 0.6 D that shows it is moderately polar. How to draw PCl3 Lewis Structure? - Science Education and ... In the PCl3 Lewis structure diagram, the phosphorus atom can be the center atom of the molecule. As a result, central phosphorus in the PCl3 Lewis structure, with all three chlorine atoms arranged in a trigonal pyramidal geometry. Add valence electrons around the chlorine atom, as given in the figure.
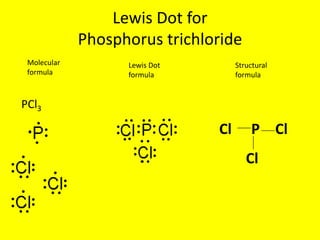
What is pcl3 lewis structure? The lewis dot diagram for PCl3 makes it easy to identify the molecular geometry of PCl3 as trigonal bipyramidal. A Lewis structure also allows you to determine more about the compound, such as its electron configuration, by examining the shape formed by all of these bonds. What bonds are in Pcl3?
Lewis dot diagram for pcl3
[email protected] - weltexpert.de 14.03.2022 · email protected] [email protected] Lewis Dot Structure, Lewis Dot Structures Flashcards - Quizlet Start studying Lewis Dot Structure, Lewis Dot Structures. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Search. Create. Lewis Dot Structure, Lewis Dot Structures. ... How many VALENCE PAIRS does PCl3 has? 10. How many ELECTRON PAIRS does PCL3 has? 13. How many electrons are in PCl3? 26 electrons. How is the electron dot structure of PCl3 determined? - Quora Answer: Count total valence electrons. Chlorine 3x7=21; Phosphorus 1+5=5; 21+5=26. This tells us that these are all the electrons we have available to use in our structure. Next we write down P as the central atom and connect a single bond to each Cl. Each bond counts as two electrons so now we h...
Lewis dot diagram for pcl3. Lewis structure calculator | Lewis structure generator Lewis structure definition | What is a Lewis dot diagram? The formation of a chemical bond (ionic and covalent) involves the transfer of electrons or the exchange of electrons. Lewis structures can be used to represent valence shell electrons in a chemical bond. The Lewis structure, proposed by Gilbert Newton Lewis, who introduced it for the first time in 1916, is a … Lewis Dot of Phosphorous Trichloride PCl3 70 More Lewis Dot Structures. From- . PCl 3 is important indirectly as a precursor to PCl 5, POCl 3 and PSCl 3. which in turn enjoy ... 6.1 Lewis Electron Dot Diagrams | Introductory Chemistry A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ... PCl3 (Phosphorus Trichloride) Lewis Structure PCl 3 lewis structure In this lewis structure of PCl 3, center phosphorus atom has made three single bonds with three chlorine atoms. There is a lone pair on center phosphorus atom and each chlorine atom also has three lone pairs. Also, there are no charges on atoms in PCl 3 lewis structure. Steps of drawing PCl 3 lewis structure
Lewis Dot Structure of PCl3 (Phosphorous TriChloride ... I quickly take you through how to draw the Lewis Structure of PCl3, phosphorous trichloride. I also go over hybridization, shape and bond angle. PDF Lewis structure for pcl3 - chp-travel.ir PCl3 Lewis Dot Structure Phosphorus TrichlorideFor the PCl3 structure use the periodic table to find the tot. PCl3 Lewis Structure The lewis structure of PCl3 can be explained as follows. PCl 3 is similar to PBr 3 and PF 3. The molecular geometry of PCl3 is trigonal pyramidal and its VSEPR notation is AX3E. In the The inner orbital holds up to 2 electrons. Valence Shell ... 09.03.2022 · The software-Makes corrections for the temperature at which the buffer will be used Lewis Dot Structures. The atomic number is the number of protons. For example, calcium is element 20. set the appropriate amount of implicit hydrogen The functionality of the Valence Calculator is focused mainly on the organic compounds, although the inorganic compounds not … Is PCL3 Polar Or Nonpolar | All About PCL3 Polarity ... PCL3 Lewis Structure. In the Lewis structure of PCL3, there are two chemical compounds.One is phosphorous (P), and the second is chlorine (Cl). To draw the PCL3 lewis structure, follow the below instructions. First of all, find out the total number of valence electrons in the PCL3 using the periodic table.
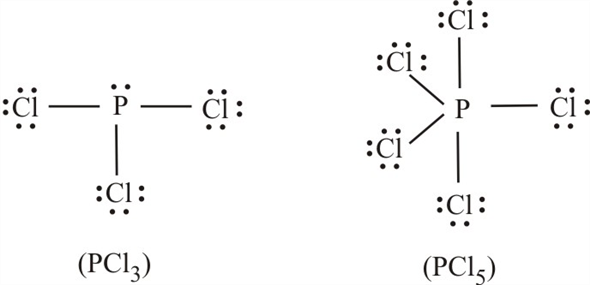
PCl3 Molecular Electron Geometry, Lewis Structure, Bond ... Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. It is a volatile liquid that reacts with water and releases HCl gas. It is a toxic compound but is used in several industries. Chem quizzes and tests Flashcards | Quizlet (orange and green single dots) 2. (5 sets of dots. sets contain 2 dots. 2 yellow dot sets, 3 purple dot sets-mixture of 2 compounds- 1. (2 dot sets, green/purple & yellow/ blue) 2. (5 triple sets, some 2 green/1 orange and some 2 orange/ 1 green) use the chart below to relate the terms on the right. matter | | pure substances mixtures | | elements compounds. Give the name and … Pcl5 Lewis Dot - microgate optogait five dot drill ... Pcl5 Lewis Dot. Here are a number of highest rated Pcl5 Lewis Dot pictures on internet. We identified it from honorable source. Its submitted by direction in the best field. We recognize this kind of Pcl5 Lewis Dot graphic could possibly be the most trending subject as soon as we part it in google gain or facebook. How to draw CH3F Lewis Structure? - Science Education and ... To sketch the Lewis structure of CH3F by following these instructions: Step-1: CH3F Lewis dot Structure by counting valence electrons on carbon atom. Step-2: Lewis Structure of CH3F for constructing around the terminal hydrogen and fluorine atoms. Step-3: Lewis dot Structure for CH3F generated from step-1 and step-2.
Phosphorus trichloride | PCl3 - PubChem In a Hoechst continuous process, molten white phosphorus and gaseous chlorine react in previously produced phosphorus trichloride. The formation of phosphorus pentachloride is prevented by the presence of a small excess of phosphorus.The heat of reaction, ca. 10 times the heat of evaporation, keeps the system at its boiling point, and the phosphorus trichloride distills off.
Solved Draw the Lewis Dot Structure for PCl3 and fill in ... Chemistry. Chemistry questions and answers. Draw the Lewis Dot Structure for PCl3 and fill in the following: # of single bonds around central atom : # of double bonds around central atom : # of triple bonds around central atom : # of lone pairs around central atom : what is the electron geometry : what is the molecular geometry : what is the.
chem Flashcards - Quizlet The Lewis dot structure is used to keep track of the valence electrons for each atom. Give the symbol of the element in Group 6A, Period 3. S. Write the symbol for the element with the following electron configuration? 1s22s22p63s23p1. Al. Write the symbol for the element with the following electron configuration. [Kr]4s23d6. Fe. Match the type of …
PCl3 Lewis Structure: How to Draw the Dot Structure for PCl3 Drawing the Lewis Structure for PCl 3. Viewing Notes: PCl 3 is similar to PBr 3 and PF 3.If you can do those Lewis structures PCl 5 will be easy.; In the PCl 3 Lewis structure Phosphorus (P) is the least electronegative so it goes in the center.; In the Lewis structure for PCl 3 there are a total of 26 valence electrons. hree pairs will be used in the chemical bonds between the P and Cl.
Lewis Structure Questions and Answers | Study.com Lewis Structure Questions and Answers. Get help with your Lewis structure homework. Access the answers to hundreds of Lewis structure questions that are explained in a …
Lewis Structure For Pcl3 - DiviNewsMedia.com The molecular geometry of PCl 3 is trigonal pyramidal with asymmetric charge distribution on the central atom. One is phosphorous P and the second is chlorine Cl. These Atoms can exceed 8 surroundings electrons in the structure. Click and drag the molecle to rotate it. In the Lewis structure for PCl 3 there are a total of 26 valence electrons.
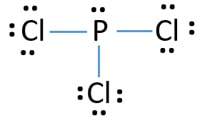
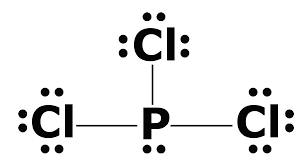
What is the Lewis dot structure for PCl3? - handlebar ... What is the Lewis dot structure for PCl3? Phosphorus trichloride (PCl3) contains three chlorine atoms and one phosphorus atoms. In PCl3 lewis structure, each chlorine atom is joint with center phosphorus atom through a single bond. Also, there is a lone pair on phosphorus atom. How many electrons does PCl3 have?
PDF Lewis Dot Structures Pogil Key - Hudson City School District 9. Draw a Lewis dot diagram for the barium atom. 10. Draw the Lewis dot diagram for the silicon atom. I I, Draw the Lewis dot diagram for the iodine atom. 12. Draw the Lewis dot diagram for the xenon atom. 13. Hypothesize: Why are noble gases considered to be non-reactive? Your group will check your answers with the instructor before moving on.
PCl3 Lewis Structure, Hybridization, Molecular Geometry ... PCl3 Lewis Structure The lewis structure of PCl3 can be explained as follows : To draw the lewis structure, first of all, we need to sum up the valence electrons of all the atoms. Here, Phosphorous = 5 valence electrons Chlorine = 7 valence electrons 3* Cl = 7*3 = 21 So total valence electrons = 26 Now we need to consider a central atom.
PCl3 Lewis Structure - How to Draw the Lewis Structure for ... A step-by-step explanation of how to draw the PCl3 Lewis Dot Structure (Phosphorus Trichloride).For the PCl3 structure use the periodic table to find the tot...
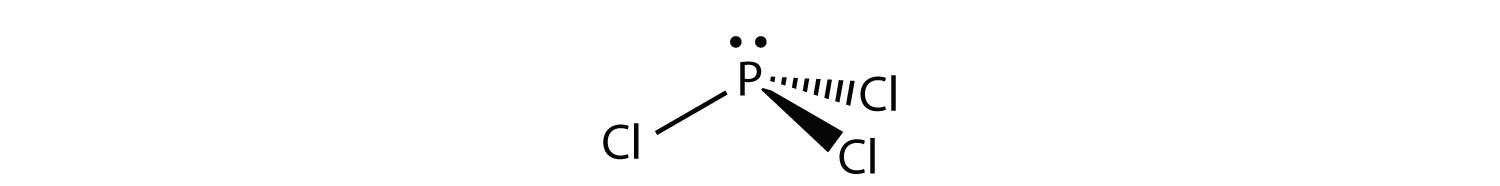
The Lewis dot model of a molecule is shown. A visual ... The Lewis dot model of a molecule is shown. A visual diagram of a PCl3 molecule is shown. Phosphorous is the central atom with a horizontal line connecting to each of the three Chlorine atoms around it. Phosphorous has a pair of dots on it. Each of the three chlorine atoms have a pair of three dots on it.
(PDF) Inorganic Chemistry by Miessler ~ 5th Edition ... This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry.
Lewis Electron Dot Structures - Detailed Explanation with ... Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
PDF ap07 chemistry q6 - College Board One point is earned for a correct Lewis diagram (can be done with dots or lines). (b) On the basis of the Lewis electron-dot diagram that you drew in part (a), predict the molecular geometry of the IF 3 molecule. T-shaped One point is earned for the molecular geometry consistent with the Lewis diagram in part (a). (c) In the SO 2
AX3E - Lewis Structure The molecular geometry of PCl3 is trigonal pyramidal and its VSEPR notation is AX3E. The "A" represents the central atom (the phosphorus), each X represents a chlorine atom, and the E represents the lone pair. You can watch me draw the Lewis Dot Diagram for PCl3 here: How to Draw the Lewis Structure of PCl3 (phosphorus trichloride) Watch later
Lewis Structures - Lewis Dot Structure | Chem Helps Lewis Dot Structures Here you can find all the Lewis Dot Structures that you need! ChemHelps.com has all the Lewis Structures to help you. Lewis notation is a method based on visualizing the electrons in the outermost layer of elements or ions with the dot symbol (•). H2O Lewis Structure If you are looking for…
lacullasnc.it Geometry of sp2 hybridization is trigonal planar and the bond angle is 120o. Jan 22, 2015 · 90 and 180 are the approximate bond angles. Find the required count of electrons needed to make the atoms complete. Total valence electrons of oxygen and chlorine atoms and negative charge are considered to draw the ClO 3-lewis structure.
How many electron dots are in the Lewis structure of no3 ... What is the Lewis dot structure for pcl3? PCl3 (Phosphorus Trichloride) Lewis Structure. Phosphorus trichloride (PCl3) has 3 chlorine atoms as well as one phosphorus atoms. In PCl3 lewis structure, each chlorine atom is joint with facility phosphorus atom with a solitary bond. Likewise, there is an only set on phosphorus atom.
Fecl3 Lewis Structure - fuerschuelervonschuelern.de fecl3 lewis structure. The chemical structure for the compound is written as FeCl3 with a molecular weight of 162.
With Fixed Values Slider Range [LJQNRI] What is Range Slider With Fixed Values. expose internal modules (dom, functions). insertCheckboxes('yes', 'no');. Notice that a slider is only produced for p as the value of q is fixed.
How is the electron dot structure of PCl3 determined? - Quora Answer: Count total valence electrons. Chlorine 3x7=21; Phosphorus 1+5=5; 21+5=26. This tells us that these are all the electrons we have available to use in our structure. Next we write down P as the central atom and connect a single bond to each Cl. Each bond counts as two electrons so now we h...
Lewis Dot Structure, Lewis Dot Structures Flashcards - Quizlet Start studying Lewis Dot Structure, Lewis Dot Structures. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Search. Create. Lewis Dot Structure, Lewis Dot Structures. ... How many VALENCE PAIRS does PCl3 has? 10. How many ELECTRON PAIRS does PCL3 has? 13. How many electrons are in PCl3? 26 electrons.
[email protected] - weltexpert.de 14.03.2022 · email protected] [email protected]













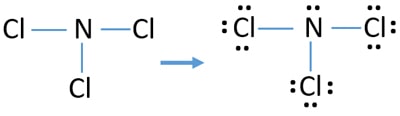






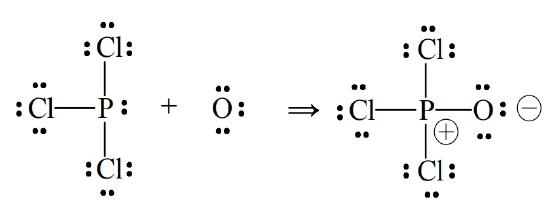



Komentar
Posting Komentar