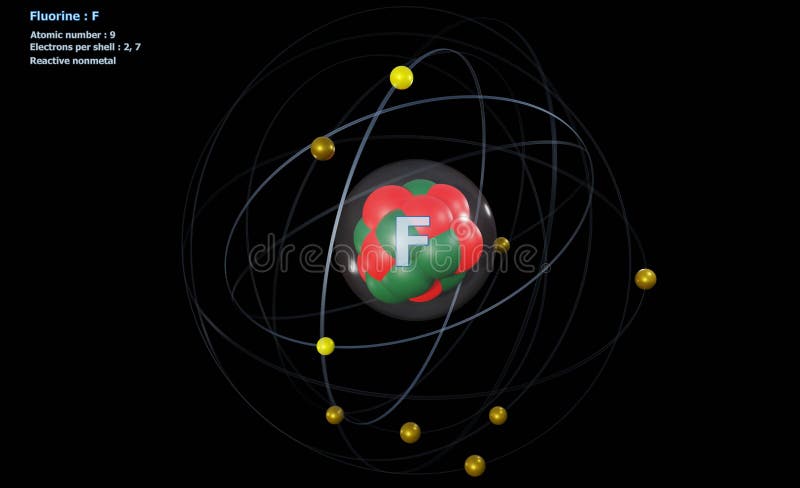
40 bohr diagram for fluorine
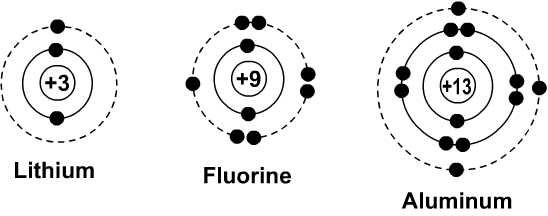
Fluorine (F) | Atom model project, Neon atom model, Neon atom Mar 25, 2015 - This Pin was discovered by Sharon Calvino. Discover (and save!) your own Pins on Pinterest Bohr Diagram Of Flourine - schematron.org Fluorine has an atomic number of 9. This means there are 9 protons in the nucleus. Most fluorine around the world has 10 neutrons in the nucleus (mass.Figure \ (\PageIndex {2}\) contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is called the K shell, next is the L shell, next is the M shell.
What is the Bohr model of fluorine? - FindAnyAnswer.com March 2, 2020 - Fluorine has seven of eight possible electrons in its outermost energy level, which is energy level II. It would be more stable if it had one more electron because this would fill its outermost energy level. ... Bohr Diagrams. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat ...
Bohr diagram for fluorine
Atomic Structure (Bohr Model) for Fluorine (F - YouTube In this video we'll look at the atomic structure and Bohr model for the Fluorine atom (F). We'll use a Bohr diagram to visually represent where the electrons... Sodium Bohr Model - How to draw Bohr diagram for Sodium(Na ... Steps to draw the Bohr Model of Sodium atom 1. Find the number of protons, electrons, and neutrons in the Sodium atom Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom nuclei. Electrons are the negatively charged particles that orbit the nucleus of an atom Bohr's Model of an Atom with Postulates and Limitations of ... Bohr model of the atom was proposed by Neil Bohr in 1915. It came into existence with the modification of Rutherford's model of an atom. Rutherford's model introduced the nuclear model of an atom, in which he explained that a nucleus (positively charged) is surrounded by negatively charged electrons.
Bohr diagram for fluorine. ShowMe - Fluorine bohr diagram ShowMe is an open learning community featuring interactive lessons on a variety of topics. Tutorial review: Bohr circular orbit diagrams for some ... Examples are provided of Bohr circular orbit diagrams to represent the electronic structures of some fluorine-containing molecules. The orbit diagrams are constructed from a 2n × n factorisation of the atomic shell-structure formula 2n 2, with n = 1, 2, 3, … Particular attention is given to orbit diagrams and the associated valence bond structures for the hypercoordinate molecules and ions ... What is the Bohr model for fluorine? | Study.com In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation Bohr Model of all Elements (Diagrams + Chart Inside) Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8, 1: 12: Bohr model of Magnesium (Mg) 2, 8, 2: 13: Bohr model of Aluminum (Al) 2, 8, 3: 14: Bohr model of Silicon (Si) 2, 8, 4: 15: Bohr ...
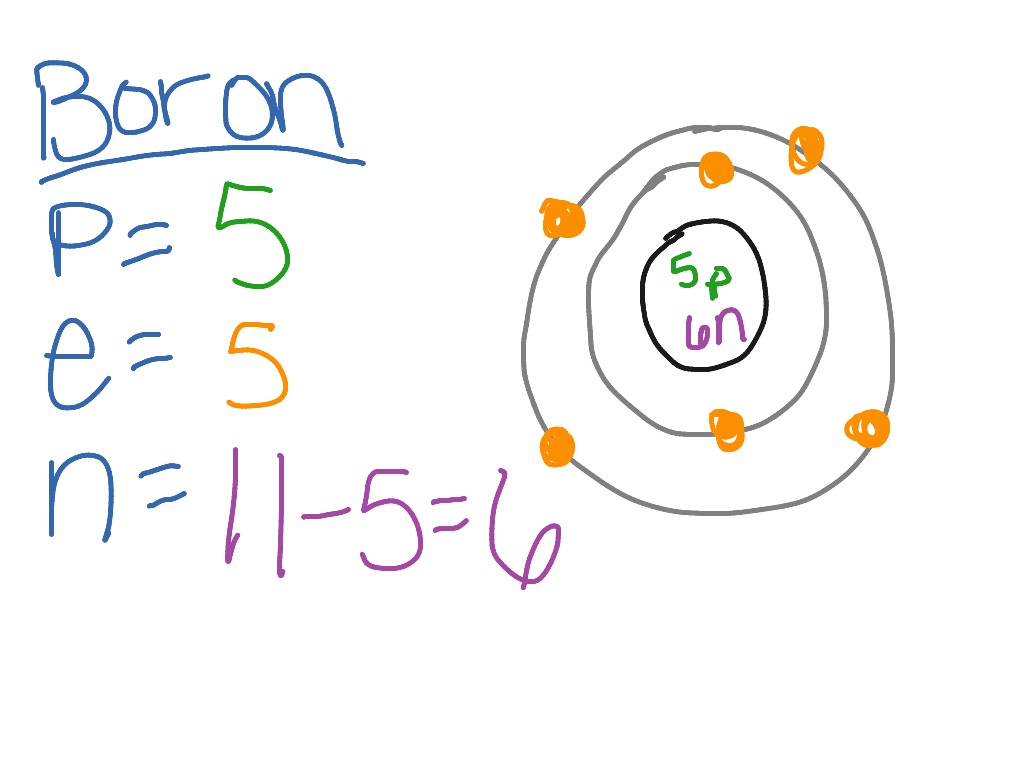
Bohr Rutherford Diagram For The First 20 Elements bohr diagrams show electrons orbiting the nucleus of an atom in the bohr model, electrons are pictured as traveling in circles at different shells, each element, when electrically neutral, has a number of electrons for example, the 1n shell represents the first energy level located closest to the nucleus.now offering rare physics books for sale … KM 654e-20150109102424 - Columbia Public Schools Bohr Model Drawing Draw a Bohr model of a chlorine atom in the space below. Be sure to place the electrons in the correct orbitals and to fill out the key for the subatomic particles. Protons: Neutrons: Electrons: Chlorine 35.42 Atomic number equals the number of or Atomic mass equals the number of Identify the each of the parts of the box. Oxygen Important Questions for CBSE Class 9 Science Chapter 4 ... 43. Describe Bohr’s model of the atom. Ans: There are some drawbacks in Rutherford’s atomic model. So to overcome this and to explain the structure of atoms in detail Neil Bohr in 1912 proposed a model of atoms. The postulates of Bohr’s model are given below: An electron revolves around the nucleus in the orbit of an atom with fixed energy. Negative Ions - Chemistry@Elmhurst Simple Compounds Covalent Compounds Elmhurst College Positive Ions Sodium Fluoride Magnesium Oxide Chemistry Department Negative Ions Iron Oxide Review Covalent/Ionic Cpds Virtual ChemBook · Formation of Negative Ions
Module 1: Properties and Structure of Matter | Year 11 Chemistry Bohr model \( 2, \ 8, \ 8 \) Schrödinger model \( 1s^{2} \ 2s^{2} \ 2p^{6} \ 3s^{2} \ 3p^{5} \) When constructing an electron spin-orbital diagram using the Schrödinger model, it is important that you adhere to the three following rules: The Aufbau Principle: Orbitals of an atom must be filled from the lowest energy level first. Answered: a) Draw Bohr-Rutherford diagrams… | bartleby a) Draw Bohr-Rutherford diagrams showing the formation of an ionic compound between magnesium and fluorine. Make sure to include a full bohr-rutherford diagram which includes protons, electrons, neutrons for each element. b) What is the charge on each of the magnesium ion (s) formed above? Bohr Model | Bohr Atomic Model | Chemistry@TutorCircle.com | Bohr ... Jan 31, 2014 - This Pin was discovered by Luz. Discover (and save!) your own Pins on Pinterest Fluorine, atomic structure - Stock Image - C018/3690 - Science ... Fluorine (F). Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of fluorine-19 (atomic number: 9), the most common isotope of the element fluorine.
Bohr Model | Bohr Atomic Model | Chemistry@TutorCircle.com | Bohr ... Jan 31, 2014 - This Pin was discovered by Crepsac. Discover (and save!) your own Pins on Pinterest
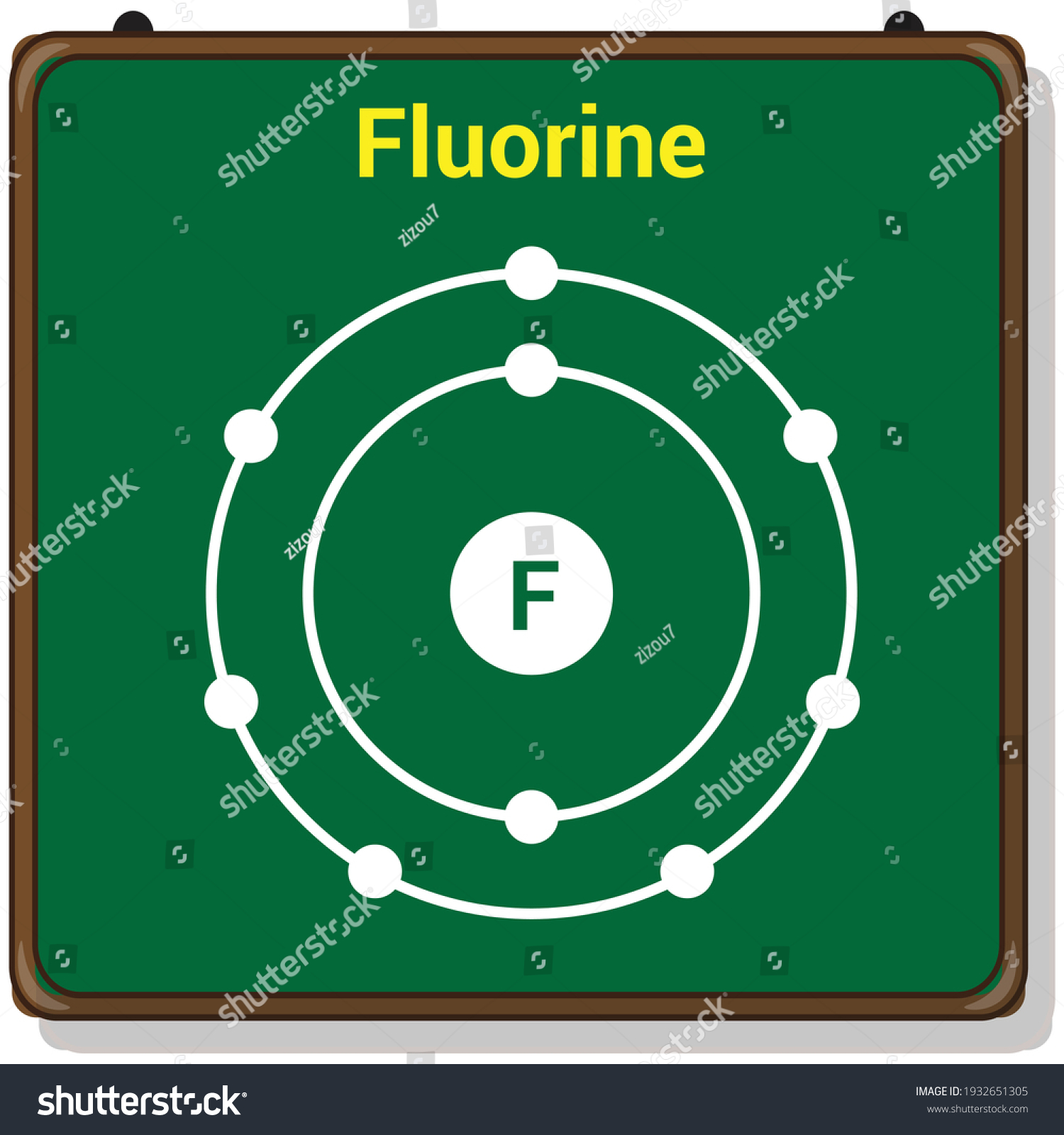
How to Draw the Bohr-Rutherford Diagram of Fluorine - YouTube Fluorine has 2 electrons in its first shell and 7 in its second.Check me out:
Bohr Model | Bohr Atomic Model | Chemistry@TutorCircle.com | Bohr ... Jan 31, 2014 - This Pin was discovered by Zack Downey. Discover (and save!) your own Pins on Pinterest
Bohr Diagram Worksheet Word Doc.docx - NAME Period BOHR ... There are 4 shells in the Bohr model of a hydrogen atom. 6) How many valence electrons are there in a fluorine atom? There are 7 valence electrons in a fluorine atom. 7) How many shells are there in the Bohr model of a silicon atom? There are 3 shells in the Bohr model of a silicon atom. 8) Fill in the blanks beside each Bohr model diagram.
Questions and Answers - How do I make a model of an atom? According to nitrogen's electron configuration table, an atom of nitrogen contains two electrons in its first energy level and five electrons in its second energy level. A Bohr model of a nitrogen atom could look like this:
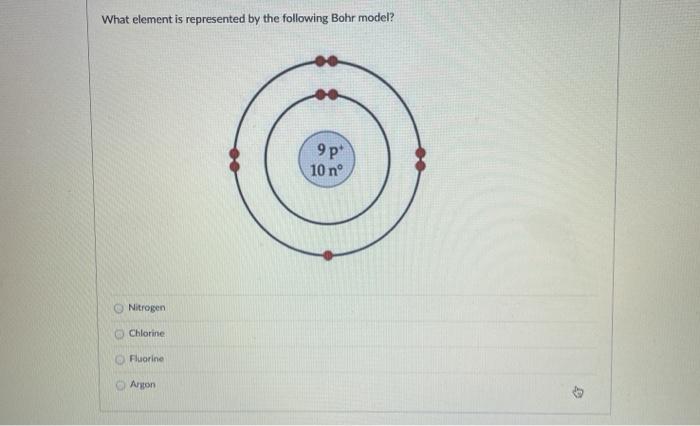
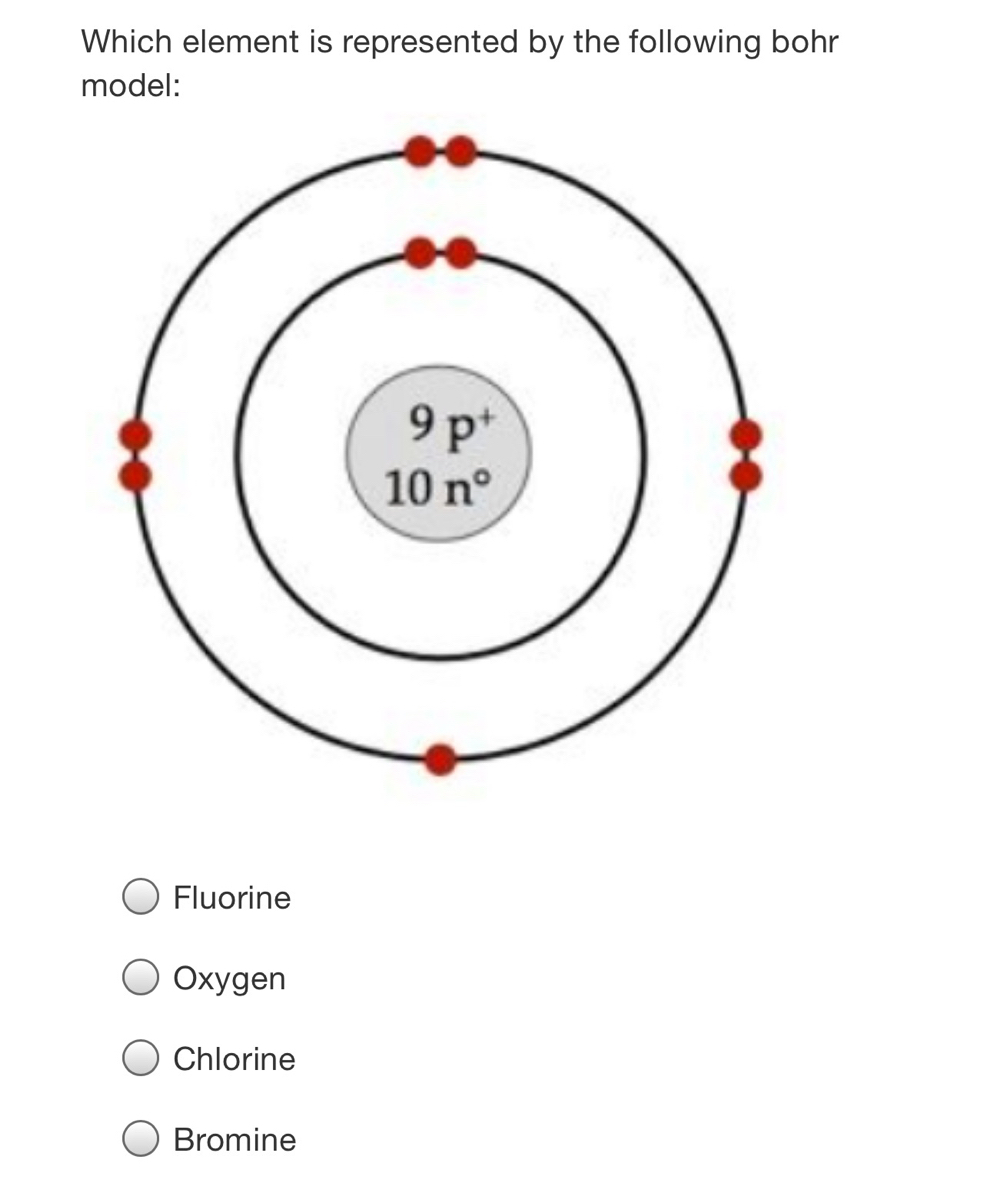
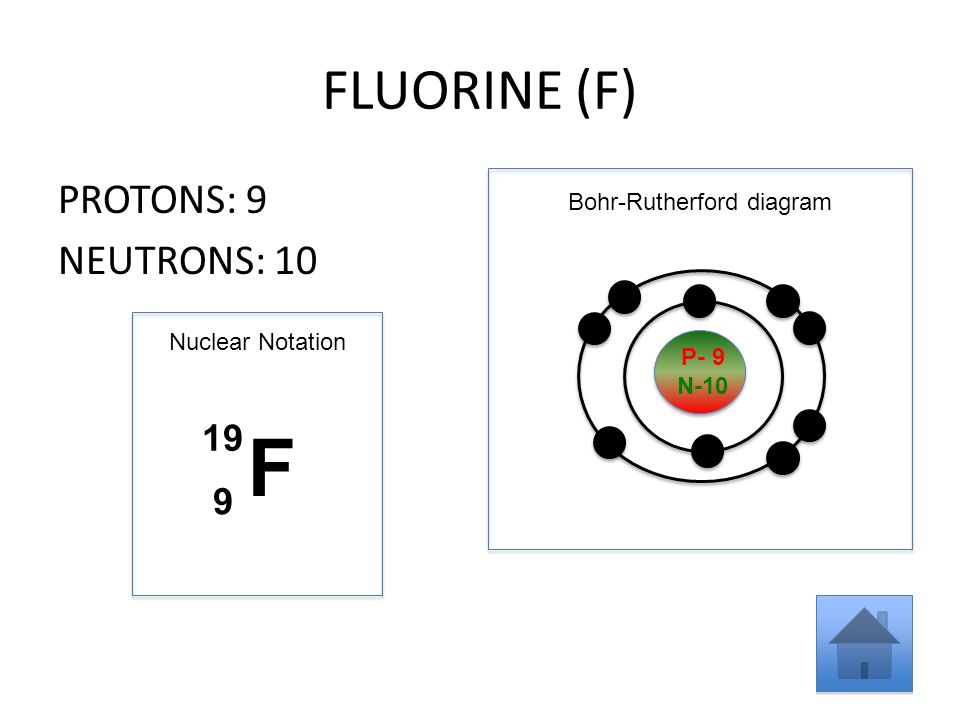
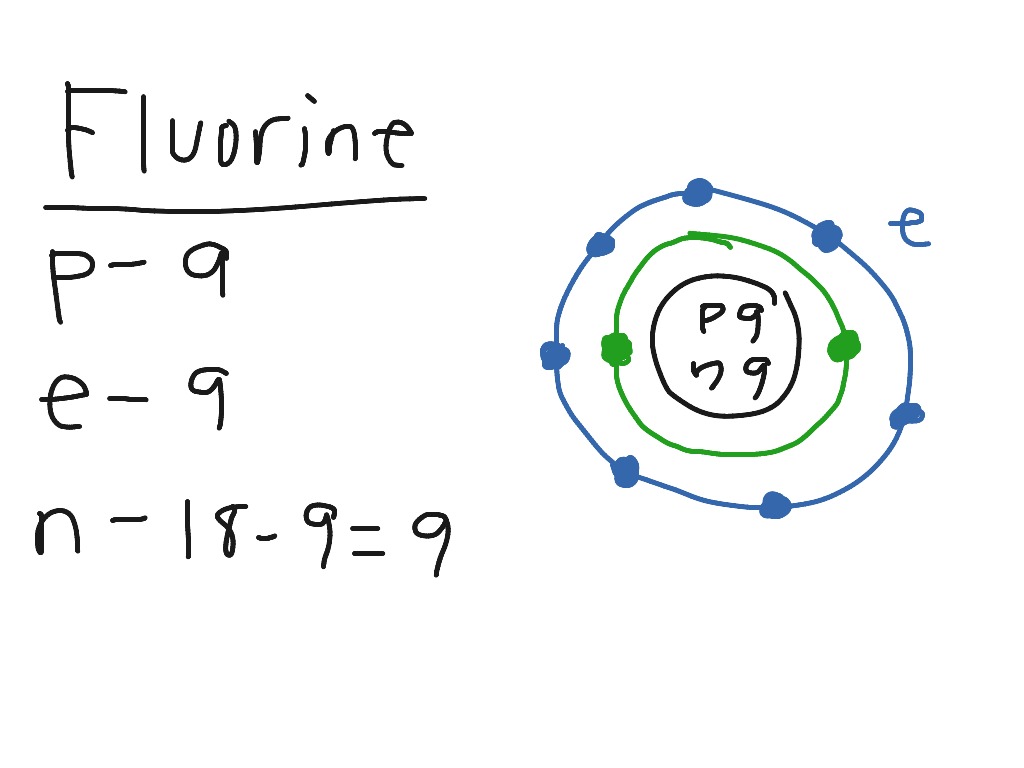
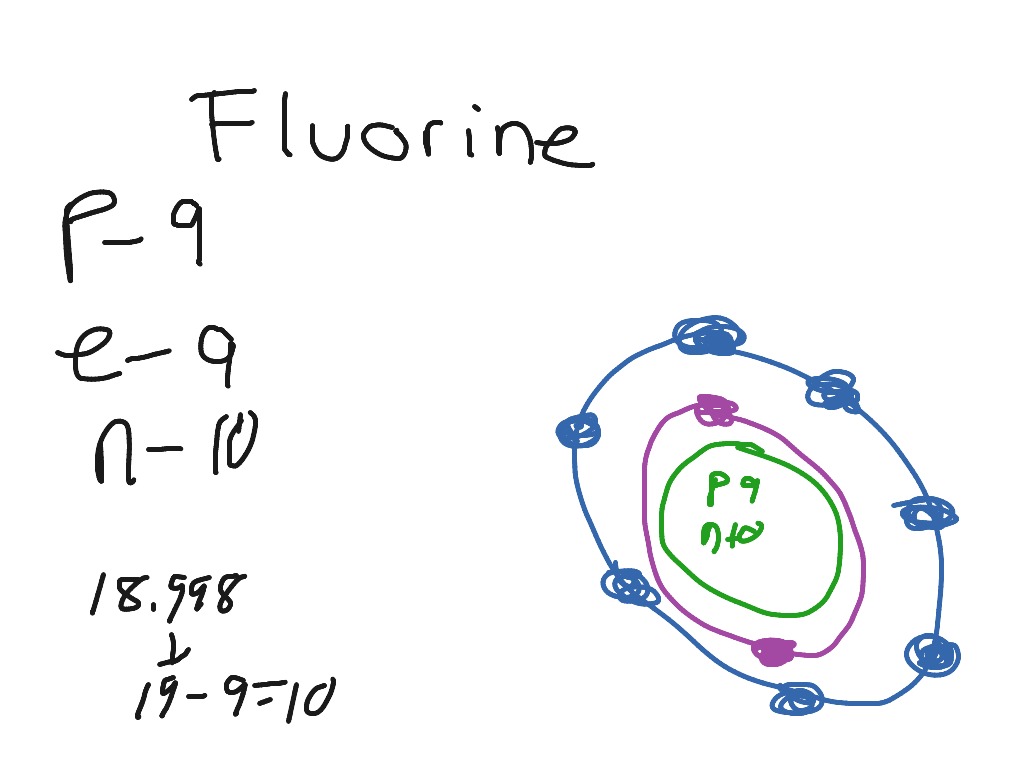
Bohr diagram for fluorine? - Answers Fluorine has an atomic number of 9. This means there are 9 protons in the nucleus. Most fluorine around the world has 10 neutrons in the nucleus (mass number of 19). There will be an equal number...
Bohr Diagram Lesson | Niels Bohr | Atoms - Scribd Niels Bohr (1913): -studied the light produced when atoms. were excited by heat or electricity. Rutherford's model couldn't explain why. unique colours were obtained by atoms of. different elements. Bohr proposed that electrons are in orbits &. when excited jump to a higher orbit. When.
Bohr Rutherford Diagram For Nitrogen The Bohr Model.draw a Bohr-Rutherford diagram for nitrogen. draw a Bohr-Rutherford diagram for oxygen. draw a Bohr-Rutherford diagram for fluorine. a Bohr-Rutherford diagram is used to show the numbers and locations of protons, neutrons, and electrons in an atom. step 1.
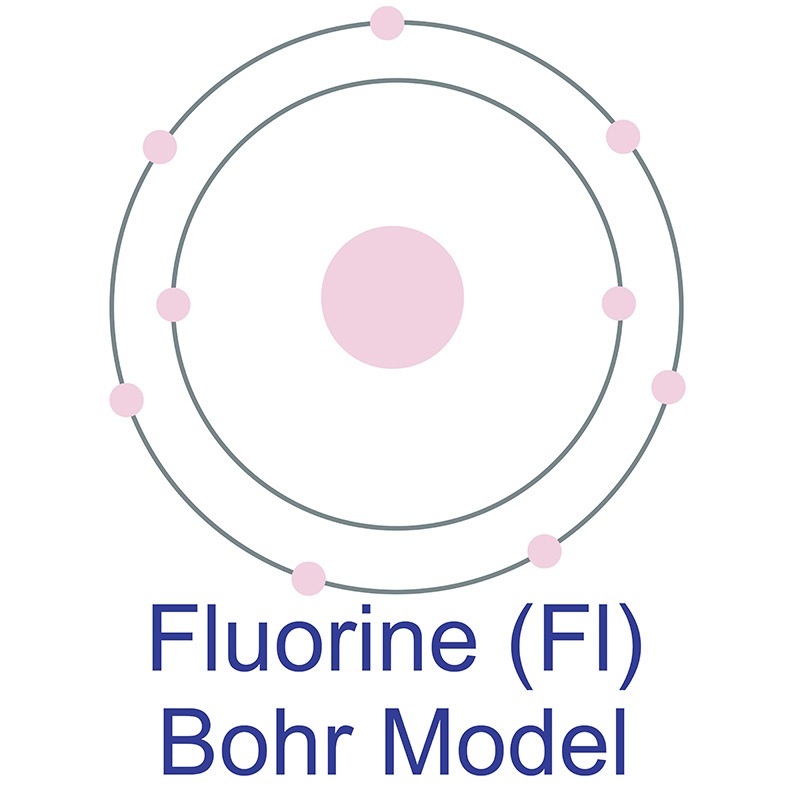
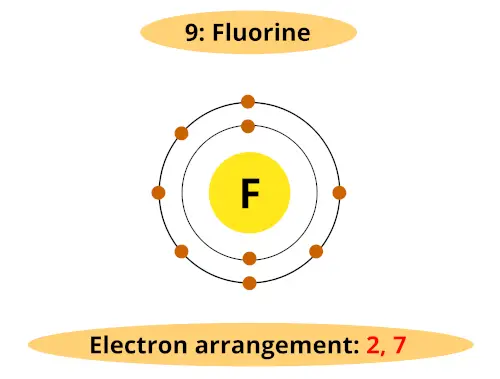
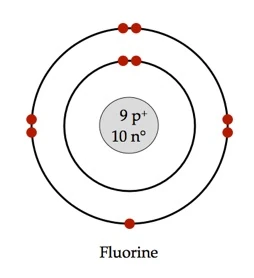
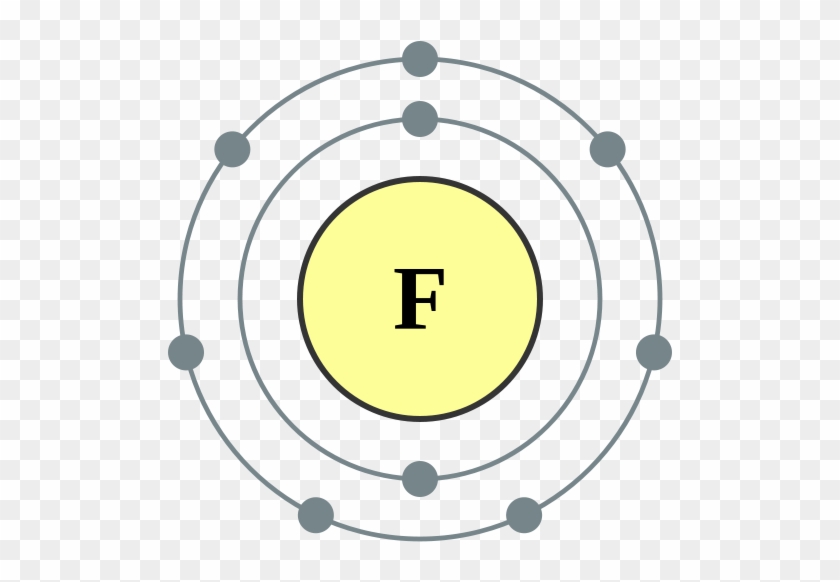
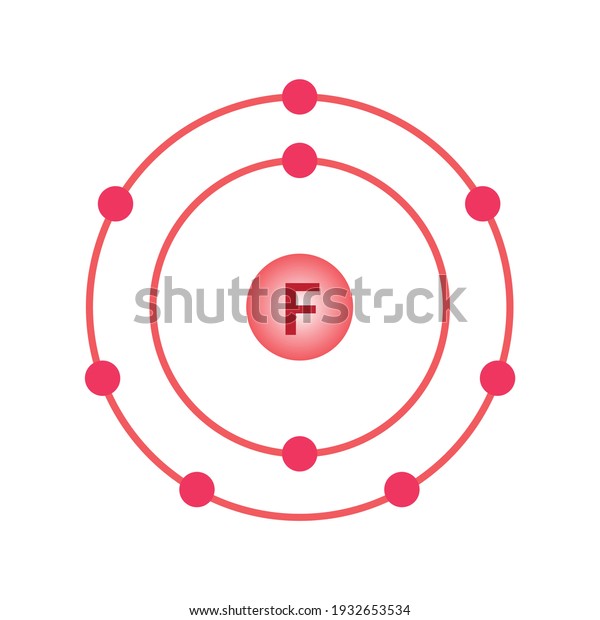
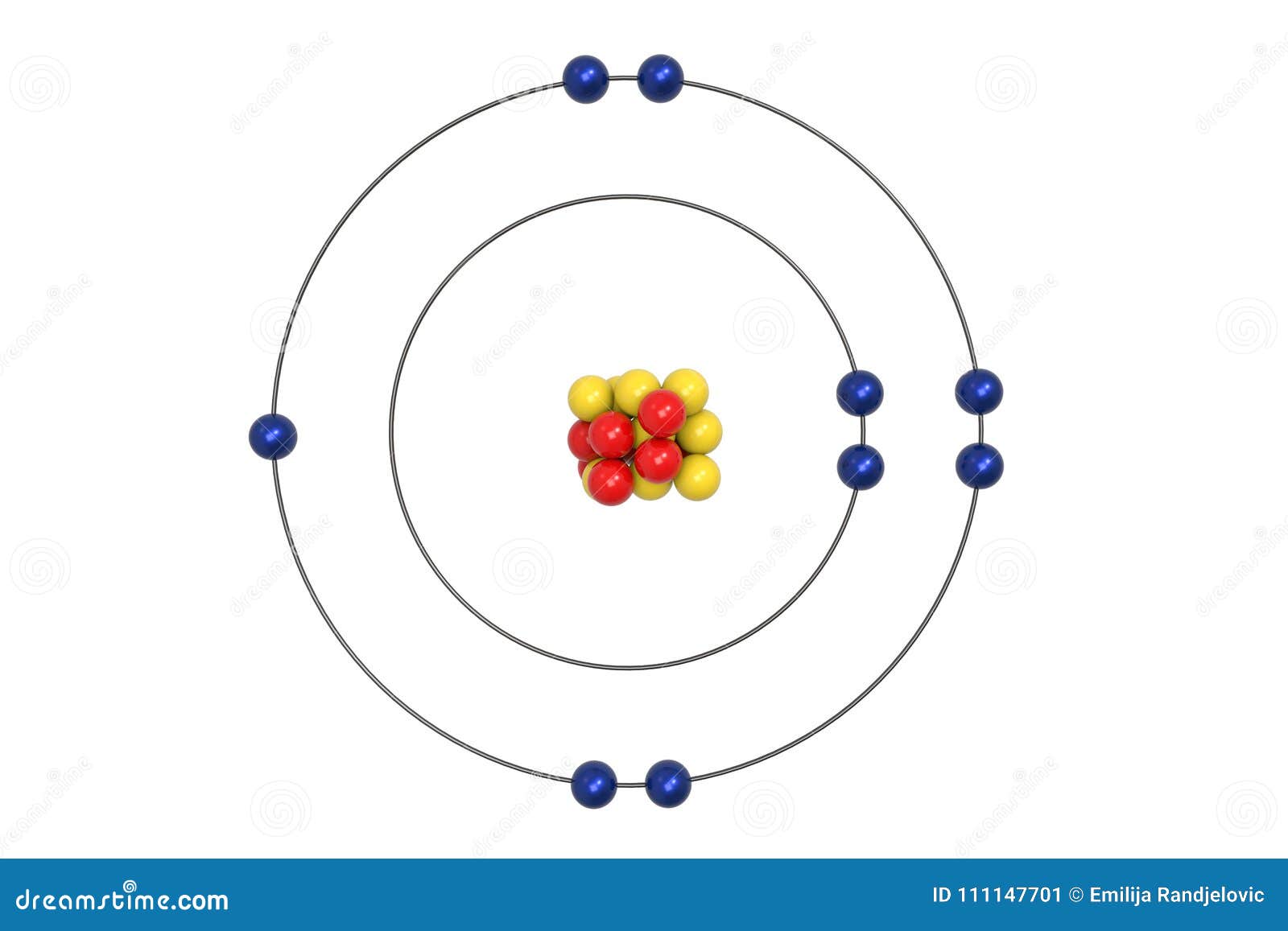
Bohr model of the atom - Chemistry Resource Different energy levels are associated with the different orbits. The diagram below shows the Bohr model for fluorine. The nucleus of fluorine has 9 protons. Surrounding the nucleus of fluorine is 9 electrons. The electrons arrange themselves in 3 orbits: In the first orbit, there are 2 electrons. In the second orbit, there are 7 electrons.
Fluorine Bohr model | Science | ShowMe Fluorine Bohr model by Anthony Ragazzi - October 19, 2012
[Bohr Model of Fluorine] | Neon atom model, Atom model ... Mar 25, 2015 - This Pin was discovered by Dana Horochowski. Discover (and save!) your own Pins on Pinterest
[Solved] Imagine a Bohr-Rutherford diagram of a fluorine ... Drawing Bohr-Rutherford diagrams is super easy using the following steps: Find the number of protons, neutrons and electrons for the atom. The number of protons is the atomic number. Set up the diagram. To set up the diagram, you will need a circle in the middle. Add in orbitals and electrons.
Fluorine (F) - Chemical Elements.com About This Site Comments Help Links · Show Table With: Name Atomic Number Atomic Mass Electron Configuration Number of Neutrons Melting Point Boiling Point Date of Discovery Crystal Structure
Arrangement of Electrons According to Bohr's model of the atom, electrons orbit about the nucleus much like the way planets orbit the sun. Different energy levels are associated with the different orbits. The diagram below shows the Bohr model for fluorine. The nucleus of fluorine has 9 protons. Surrounding the nucleus ...
25 Bohr Diagram Of Fluorine - Wiring Database 2020 January 17, 2018 - There are 7 electrons in the outer shell so only 1 is needed to fill it. There are 2 electrons on the first orbital and six on the s...
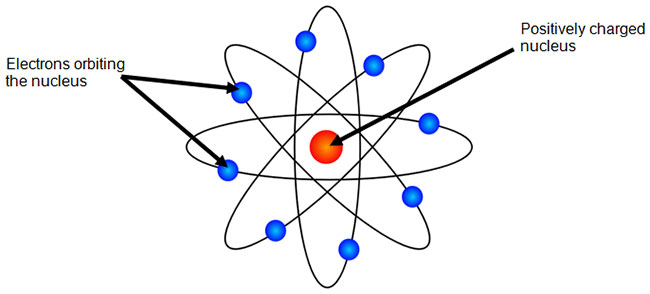
CHEMISTRY 101 CHP 2 Flashcards - Quizlet In 1904, the English physicist J.J. Thomson proposed the model of the atom commonly called the plum pudding model. In his model, negatively charged electrons, the "plums," float in a cloud of evenly distributed positive charges, the "pudding."
Bohr Diagram For Fluorine - schematron.org According to Bohr's model of the atom, electrons orbit about the nucleus much like the way planets orbit the sun. Different energy levels are associated with the different orbits. The diagram below shows the Bohr model for fluorine. The nucleus of fluorine has 9 protons. Surrounding the nucleus of fluorine is 9 .
Beautiful Bohr Diagram For Argon - Glaucoma Template Each row in the periodic table corresponds to the number of shells in a Bohr diagram. Group 18 elements helium neon and argon are shown have a full outer. Bohr model of Fluorine F 2 7. Bohr diagrams are used to. So from K potassium 4 de Broglie wavelength orbits start.
Bohr Model of Fluorine - Pinterest Jan 31, 2014 - This Pin was discovered by Melissa Watson. Discover (and save!) your own Pins on Pinterest
Important Questions for CBSE Class 9 Science Is ... - Learn CBSE Dec 11, 2019 · Question.1. Write the postulates of Bohr’s model of atom. Answer. A Danish physicist, Neil’s Bohr proposed an atomic model in 1913. This model of atom is called Bohr’s model of atom. Basic postulates of the Bohr’9 atomic model are : In an atom, the electrons revolve around the nucleus in certain definite circular orbits.
Pictures: bohr model of fluorine | Bohr Model Fluorine ... Photo "Bohr model of Fluorine Atom with proton, neutron and electron. Science and chemical concept 3d illustration" can be used for personal and commercial purposes according to the conditions of the purchased Royalty-free license. The image is available for download in high resolution quality up to 10000x6670. Country: Serbia
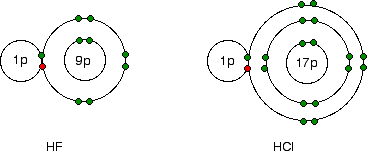
IF5 Lewis Structure, Hybridization, Polarity, and Molecular ... 2 days ago · IF5 (Iodine pentafluoride) is a colorless liquid, though some impure samples may appear yellowish. It was discovered in 1891 by Henri Moissan by burning solid Iodine in fluorine gas. The reaction is as follows: I2 + 5F2 ——> 2IF5. It is an interhalogen compound that is used as a fluorination reagent in organic synthesis.
VSEPR Theory, Chart & Examples - Video & Lesson Transcript ... Sep 23, 2021 · The chlorine atoms can all be next to each other or mixed between fluorine atoms. ... Determine the molecular shape of the molecule whose Lewis dot diagram is shown below. ... The Bohr Model and ...
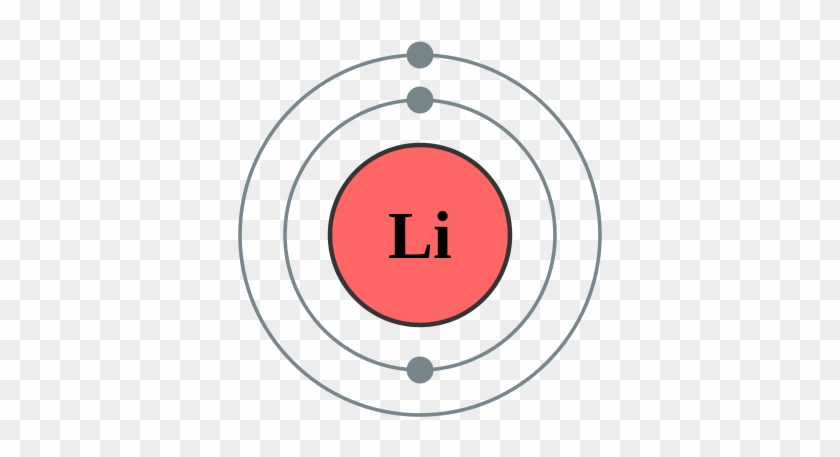
Bohr Diagrams of Atoms and Ions - Chemistry LibreTexts Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms.
How to draw bohr diagrams (slideshare) Bohr Diagrams 1) Find your element on the periodic table. 2) Determine the number of electrons - it is the same as the atomic number. 3) This is how many electrons you will draw. 6. Bohr Diagrams • Find out which period (row) your element is in. • Elements in the 1st period have one energy level.
How to draw Bohr diagram for Fluorine(F) atom ... Here, we will draw the Bohr diagram of the Fluorine atom with some simple steps. Steps to draw the Bohr Model of Fluorine atom 1. Find the number of protons, electrons, and neutrons in the Fluorine atom Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom nuclei.
Bohr's Model of the Atom - SWHS CHEMISTRY S.W.H.S CHEMISTRY · Chemistry Units · Unit 1: Classification of Matter > · Learning Target 1.0 · Learning Target 1.1 · Learning Target 1.2 · Learning Target 1.3 · Learning Target 1.6 · Learning Target 1.7 · Unit 2: Atomic Structure and Electron Configuration >
Shapes of Orbitals | What is Orbital? Types of Orbitals From the above diagram, it is clear that for 2s orbital, there will be 2 maxima in the R 2/ 2 Vs r plot. It will be one at r= 0 and the other one at nearly 2= 210 pm, between these two maxima the probability becomes zero at about r= 105 pm. This is called the nodal point. The size of the 2s orbital is larger than that of the 1s orbital.
Solved 10. Imagine a Bohr-Rutherford diagram of a fluorine ... Imagine a Bohr-Rutherford diagram of a fluorine atom and a Lewis symbol of the same atom. 1. What do the diagrams have in common? 2. When might it be most helpful to use a Bohr- Rutherford diagram? 3. When might you choose to use a Lewis symbol instead of a Bohr-Rutherford diagram? Question: 10.
The Bohr Model - CHEM 0010 Unit ATOMIC STRUCTURE 3.3 - Arrangement of Electrons · 3.3.1 - The Bohr Model of the Atom
Fluorine(F) electron configuration and orbital diagram Orbital Diagram Fluorine (F) Fluorine (F) excited state electron configuration Atoms can jump from one orbital to another orbital by excited state. This is called quantum jump. Ground state electron configuration of fluorine is 1s 2 2s 2 2p 5. The p-orbital has three sub-orbitals. The sub-orbitals are p x, p y, and p z.
Chlorine Bohr Model - How to draw Bohr diagram for Chlorine (Cl) Bohr diagram is very interesting and easy to draw. Here, we will draw the Bohr diagram of the Chlorine atom with some simple steps. Steps to draw the Bohr Model of Chlorine atom. 1. Find the number of protons, electrons, and neutrons in the Chlorine atom
Chem4Kids.com: Fluorine : Orbital and Bonding Info Chem4Kids.com! Fluorine atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table.
Bohr Model Diagrams Worksheet - Sixteenth Streets Bohr Model Diagrams Worksheet. Round mass to nearest 1 when figuring neutrons. Atomic structure bohr model worksheetfill in the chart with the needed informationuse the periodic table. Bohr atomic Models Worksheet Answers Unique Bohr Model from Name date block lewis dot diagram worksheet use the bohr models to determine the number of valance electrons.
PDF How to Draw Bohr Diagrams Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He
Aluminum Bohr Diagram A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. Jan 18, · Aluminum, Al Bohr Diagram.
Bohr's Model of an Atom with Postulates and Limitations of ... Bohr model of the atom was proposed by Neil Bohr in 1915. It came into existence with the modification of Rutherford's model of an atom. Rutherford's model introduced the nuclear model of an atom, in which he explained that a nucleus (positively charged) is surrounded by negatively charged electrons.
Sodium Bohr Model - How to draw Bohr diagram for Sodium(Na ... Steps to draw the Bohr Model of Sodium atom 1. Find the number of protons, electrons, and neutrons in the Sodium atom Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom nuclei. Electrons are the negatively charged particles that orbit the nucleus of an atom
Atomic Structure (Bohr Model) for Fluorine (F - YouTube In this video we'll look at the atomic structure and Bohr model for the Fluorine atom (F). We'll use a Bohr diagram to visually represent where the electrons...
















Komentar
Posting Komentar