42 isobaric pv diagram
What is Isentropic Expansion - Isentropic Compression ... 22.05.2019 · Example: Isentropic Expansion in Gas Turbine P-V diagram of an isentropic expansion of helium (3 → 4) in a gas turbine. Assume an isentropic expansion of helium (3 → 4) in a gas turbine.Since helium behaves almost as an ideal gas, use the ideal gas law to calculate outlet temperature of the gas (T 4,is).In this turbines the high-pressure stage receives gas … PDF Thermodynamic systems - Northeastern University Isobaric process: p = constant V P V1 1 2 pV 2 =nRT 2 pV 1 =Nk BT1 W pdV p V V p V V V → =∫ =( 2 −1) =∆ 2 1 V2 C T Q C T T p p = ∆ = ( 2 −1) (C P: heat capacity at constant pressure) C T p V U Q W =P∆−∆ →∆ = − During an isobaric expansion process, heat enters the system. Part of the heat is used by the system to do work ...
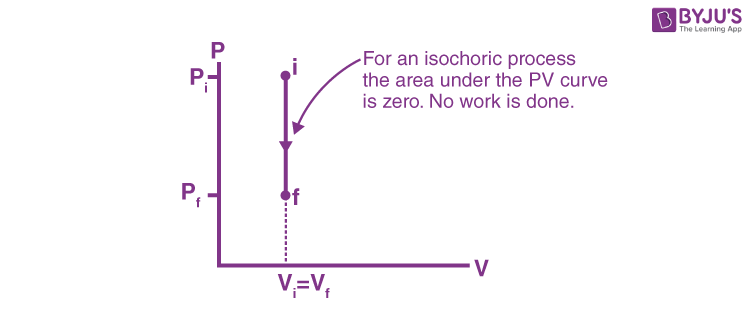
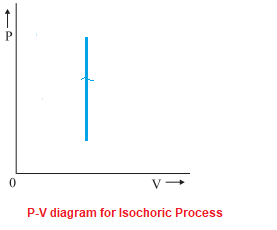
PV Diagrams: Definition & Examples | StudySmarter Constant volume (isometric or isochoric) processes and constant pressure (isobaric) processes follow a straight line in PV diagrams. You can see these processes below. Constant volume (isometric or isochoric) processes In a process with constant volume (isometric or isochoric), lines will be straight, vertical lines (see diagram 6).
Isobaric pv diagram
Which process is represented by the PV diagram? A. the ... The answer would be A. Isobaric Process. Explanation: Isobaric process is a process where the pressure inside a system remains unchanged. In the Pressure Volume graph given, you can see that the pressure (y axis) remains constant with an increasing volume ( x axis). An example of this would be heating a container with a movable piston. Draw the PV diagram for: (a) Isothermal process (b ... `PV = n RT` holds good for A) Isobaric process B) Isochoric process C) Isothermal process D) Adiabatic process asked Jun 15, 2019 in Physics by SarojSahu ( 90.5k points) class-11 Thermodynamics, PV Diagrams, Internal Energy ... - YouTube This physics video tutorial explains the concept of the first law of thermodynamics. It shows you how to solve problems associated with PV diagrams, interna...
Isobaric pv diagram. Thermodynamic processes : isothermal, adiabatic, isochoric ... Changes in pressure and gas volume in the isobaric process are illustrated by the graph below: First, system volume = V1(small volume). Because pressure is kept constant so after heat is added to the system, the system expands and does work on the environment. After working on environment, system volume changes to V2(system volume increases). Pressure volume diagram - Energy Education The PV diagram models the relationship between pressure (P) and volume (V) for an ideal gas.An ideal gas is one that never condenses regardless of the various changes its state variables (pressure, volume, temperature) undergo. In addition, the processes plotted on PV diagrams only work for a closed system (in this case the ideal gas), so there is no exchange of … Isobaric, Isochoric, Isothermal, and Adiabatic Processes We can represent these differences using a pressure volume diagram, of which there are four different types, depending on the type of process that has occurred: isobaric, isochoric, isothermal, or adiabatic. A pressure volume diagram has volume on its x-axis and pressure on its y-axis. Isobaric Process - Ideal Gas Equation - Nuclear Power On a p-V diagram, the process occurs along a horizontal line (called an isobar) with the equation p = constant. Let assume an isobaric heat addition in an ideal gas. In an ideal gas, molecules have no volume and do not interact. According to the ideal gas law, pressure varies linearly with temperature and quantity and inversely with volume.
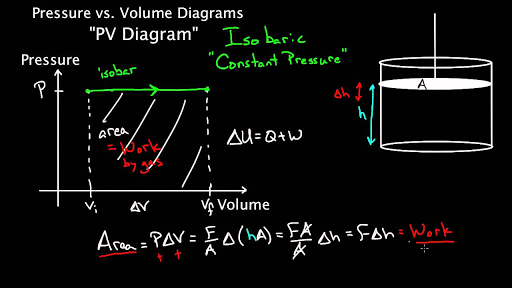
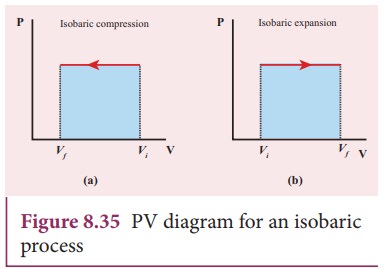
Isobaric Process - Definition, Examples, Formula - BYJUS This process is shown on the P - V diagram as the horizontal line 1 → 2. We call this an isobaric expansion. As isobaric compression occurs if the gas is cooled, lowering the piston. An isobaric process appears on a P - V diagram as a horizontal line. Isobaric Process Example Isobaric Process Paths on PV and TS Diagrams - Learn Thermo Ch 7, Lesson E, Page 10 - Isobaric Process Paths on PV and TS Diagrams. When delta equals zero in a polytropic process, the process is isobaric. The equation for a polytropic path with delta equal to zero is just P = C. What Is The Shape Of PV Diagram For Isochoric Process? What is an isobaric process give its PV diagram? PV Diagrams and their Relationship to Work Done on or by a Gas. A process by which a gas does work on a piston at constant pressure is called an isobaric process. Since the pressure is constant, the force exerted is constant and the work done is given as PΔV. ... Drawing an Isobaric Process on a Pressure-Volume Diagram PV diagram At a constant pressure of 100 kPa, 300 kJ of heat is supplied to the air. If the temperature of the air increases from 250 K to 325 K, the process will be drawn on the pressure-volume ...
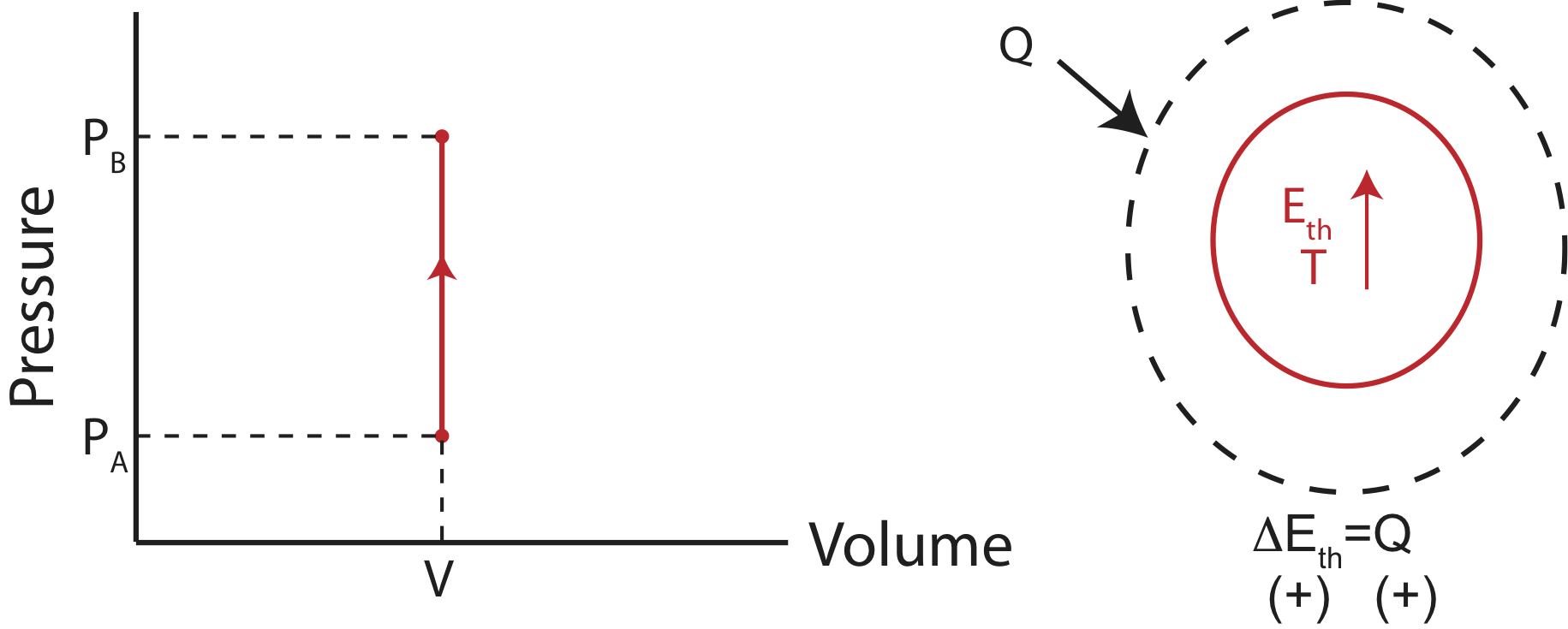
Isochoric Process - Definition, Example, Formula, P-V Diagram This is represented by a vertical line on a P - V diagram. Since the volume does not change, Whether the pressure change is positive or negative, the work done by the gas in an isochoric process is zero. Work done by a gas in a process in which PV n = constant, where n is also constant. Also Read: Thermodynamics What is Isobaric Curve - Isobar - pV Diagram - Definition An isobaric process is a thermodynamic process, in which the pressure of the system remains constant (p = const). The heat transfer into or out of the system does work, but also changes the internal energy of the system. On a p-V diagram, the process occurs along a horizontal line (called an isobar) that has the equation p = constant. What is Isobaric Process - Definition - Thermal Engineering Isobaric Process - Ideal Gas Equation See also: What is an Ideal Gas On a p-V diagram, the process occurs along a horizontal line (called an isobar) that has the equation p = constant. Let assume an isobaric heat addition in an ideal gas. In an ideal gas, molecules have no volume and do not interact. In the following pressure - volume diagram, the isochoric ... Click here👆to get an answer to your question ️ In the following pressure - volume diagram, the isochoric, isothermal and isobaric parts, respectively, are
Adiabatic Process - an overview | ScienceDirect Topics The Brayton cycle consists of the following processes: adiabatic compression 1 → 2, isobaric heat addition 2 → 3, adiabatic expansion 3 → 4, and isobaric heat removal. A p-V diagram of the cycle is depicted in Fig. 5.2. For the Brayton cycle, we have p 2 = p 3, p 4 = p 1, CR = V 4 /V 2, and PR = p 2 /p 4.
PV diagrams - part 1: Work and isobaric processes ... Visit us ( ) for health and medicine content or ( ) for MCAT...
PV diagrams - part 1: Work and isobaric processes Isobaric means constant pressure. So how do you represent this on a PV DIagram? Well, if you wanna maintain constant pressure, you can't go up or down, because if I were to go up, my pressure would be increasing. If I were to go down, my pressure would be decreasing. The only option available is to go along a horizontal line.
What does the area under horizontal lines of PV diagram in ... Answer (1 of 2): If you have horizontal lines on a P-V graph, then the pressure is constant, but the volume is changing. If I find the area under the curve, I'm looking at totals of their product. That product is equal to the work done to change the volume, so the total area is going to represent...
P-V diagram for different thermodynamic process - MechoMotive Therefore , PV diagram for Isochoric Process will be straight line. and from it we can conclude that the change in Volume is zero , therefore it's work done will also be zero. (b) Isobaric Process :
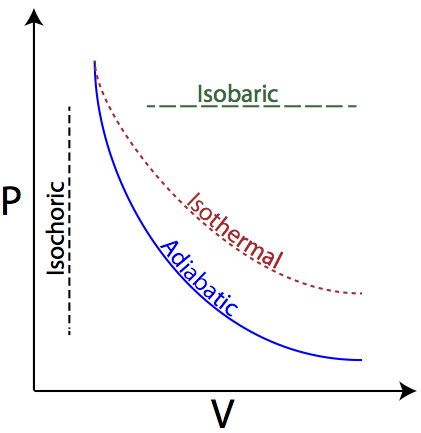
Isochoric, isobaric, isothermal and adiabatic processes Isochoric, isobaric, isothermal and adiabatic processes. At A2 we take both isothermal and adiabatic to mean at constant temperature. In fact, isothermal means the temperature remains constant, and adiabatic means that there are no heat transfer processes. All four processes can be presented on a p-V graph (the blue lines are isotherms ...
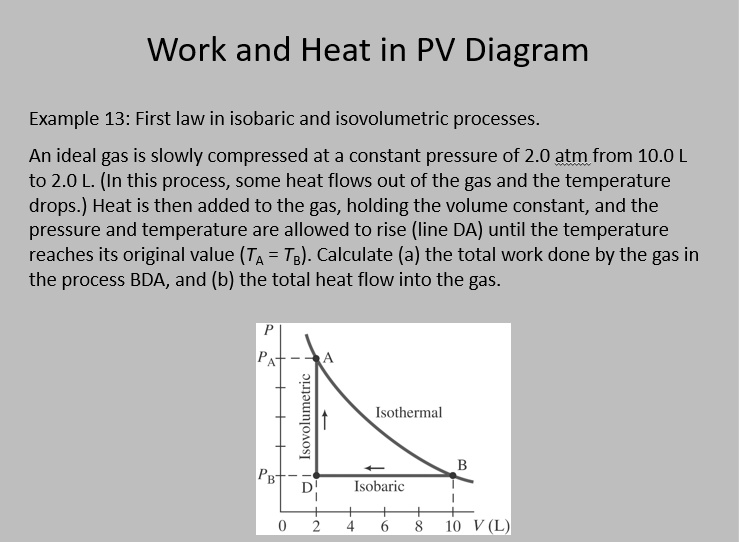
Isobaric thermodynamics processes - problems and solutions Isobaric thermodynamics processes - problems and solutions. by physics.gurumuda.net. 1. PV diagram below shows an ideal gas undergoes an isobaric process. Calculate the work is done by the gas in the process AB. Known : Pressure (P) = 5 x 105 N/m2. Initial volume (V1) = 2 m3. Final volume (V2) = 6 m3.
Isobaric Curve - Isobar - pV Diagram - Nuclear Power An isobaric process is a thermodynamic process, in which the pressure of the system remains constant (p = const). The heat transfer into or out of the system does work, but also changes the internal energy of the system. On a p-V diagram, the process occurs along a horizontal line (called an isobar) that has the equation p = constant.
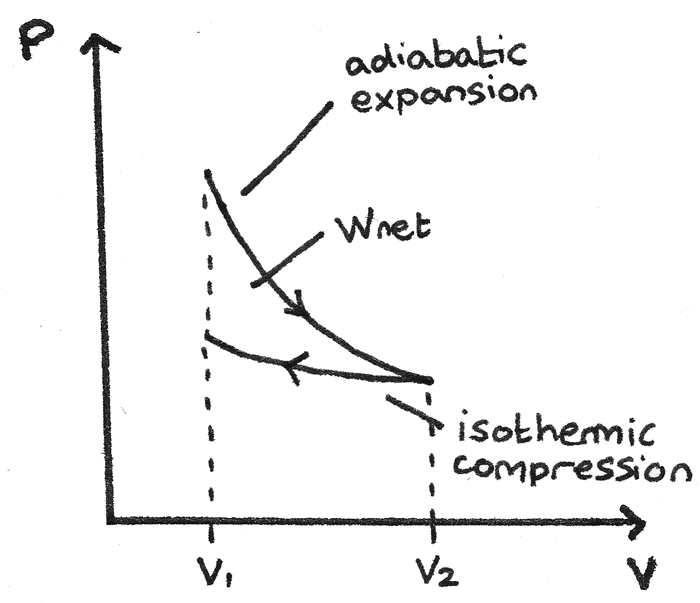
PV diagrams - part 2: Isothermal, isometric, adiabatic ... So on a PV diagram, an isothermal process is gonna look something like this, it's gonna curve like 1/x and it can be an isothermal expansion if volume increases or an isothermal compression if volume decreases. So the actual shape of the line drawn on a PV diagram for an isothermal process is sometimes called an isotherm and they look like that.
P-V Diagram | bartleby A P-V diagram is a very important tool of the branch of physics known as thermodynamics, which is used to analyze the working and hence the efficiency of thermodynamic engines. As the name suggests, it is used to measure the changes in pressure (P) and volume (V) corresponding to the thermodynamic system under study.
Pressure-Volume Diagrams - The Physics Hypertextbook isobaric constant pressure "bar" comes from the greek word for heavy: βαρύς [varys] examples: weighted piston, flexible container in earth's atmosphere, hot air balloon; PV graph is a horizontal line
PDF Lesson 42c: PV Diagrams - Studyphysics A PV diagram is a graph of Pressure as a function of Volume. There are four different situations that you can expect to see shown in PV diagrams: 1. Isobaric: the gas is held at a constant pressure 2. Isochoric: the gas is held at a constant volume 3. Isothermal: the gas is held at a constant temperature 4. Adiabatic: No heat flows in or out of ...
Isobaric process - Wikipedia An isobaric process is shown on a P–V diagram as a straight horizontal line, connecting the initial and final thermostatic states. If the process moves towards the right, then it is an expansion. If the process moves towards the left, then it is a compression. Sign convention for work. The motivation for the specific sign conventions of thermodynamics comes from early development …
P-V and T-S Diagrams - NASA 13.05.2021 · This plot is called a T-s diagram. Lines of constant pressure curve from the lower left to upper right on a T-s diagram. A constant pressure process is called an isobaric process and this type of process occurs in the combustor of a gas turbine engine. During an isentropic process there is no change in the entropy of the system and the process is reversible. An isentropic …
Thermodynamics, PV Diagrams, Internal Energy ... - YouTube This physics video tutorial explains the concept of the first law of thermodynamics. It shows you how to solve problems associated with PV diagrams, interna...
Draw the PV diagram for: (a) Isothermal process (b ... `PV = n RT` holds good for A) Isobaric process B) Isochoric process C) Isothermal process D) Adiabatic process asked Jun 15, 2019 in Physics by SarojSahu ( 90.5k points) class-11
Which process is represented by the PV diagram? A. the ... The answer would be A. Isobaric Process. Explanation: Isobaric process is a process where the pressure inside a system remains unchanged. In the Pressure Volume graph given, you can see that the pressure (y axis) remains constant with an increasing volume ( x axis). An example of this would be heating a container with a movable piston.

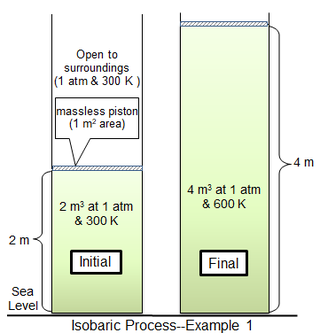

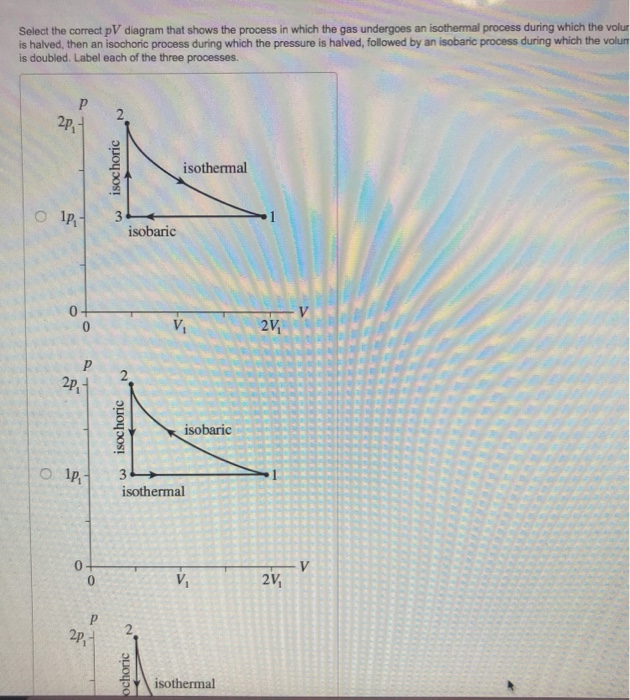



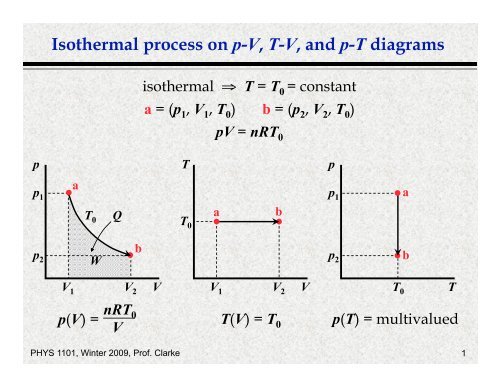

![University Thermodynamics: PV Diagrams] Why is this phase ...](https://preview.redd.it/l0b4qmuln0z41.png?auto=webp&s=ef9a64380ff6389c844c4c2523f31fc9d48f171c)










Komentar
Posting Komentar